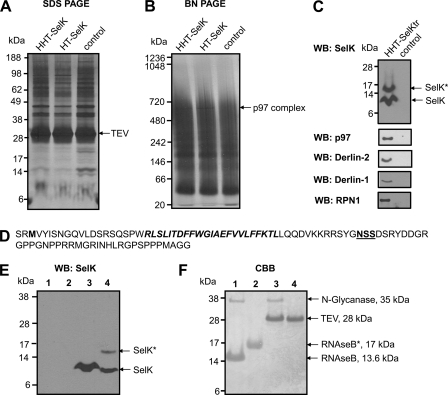
FIGURE 4.
Isolation and identification of SelK-associated proteins. A–C, HEK 293 cells (control) and HEK 293 cells transfected with the constructs coding for HHT-SelK, HT-SelK, or HHT-SelKtr were lysed in 1.8% digitonin, and the lysates were subjected to immunoprecipitation with anti-HA beads. Bound material was eluted from the beads with TEV protease and separated on SDS-PAGE (A) or Blue Native PAGE (B) or subjected to Western blotting (WB) analysis with the indicated antibodies (C). Proteins were visualized by silver staining (A and B). Migration of TEV protease and a band that was cut and subjected to LC-MS/MS (marked as TEV and p97 complex) is shown on the right for A and B, respectively. Migration of SelK and the glycosylated SelK (SelK*) is displayed by arrows in C. D, sequence of the truncated form of SelK (Sec-to-Stop mutation) that occurs following expression of the construct coding for HHT-SelKtr, protein isolation, and treatment with TEV protease. Two amino acid residues in the N-terminal region upstream of the N-terminal methionine residue (shown in bold type) are those that remain after the cleavage with TEV protease; the transmembrane domain is shown in bold type; and the Asp-Ser-Ser (NSS) sequon is displayed in bold and underlined type. E, Western blotting analysis with SelK antibodies of samples prepared in the deglycosylation assay. Lane 1, 5 μg of RNase B treated with N-glycanase (control for deglycosylation); lane 2, 5 μg of RNase B; lane 3, sample prepared during affinity purification of the truncated form of SelK (as for C) and treated with N-glycanase; lane 4, untreated sample from lane 3. Migration of deglycosylated SelK (SelK) and glycosylated SelK (SelK*) is shown on the right. SelK mobility in treated and untreated samples is different because of detergent addition in the glycosylation assay. F, Coomassie Brilliant Blue (CBB) staining of the same membrane. Migration and mass of N-glycanase, TEV protease, glycosylated RNase B (RNAseB*), and deglycosylated RNase B are pointed out on the right.