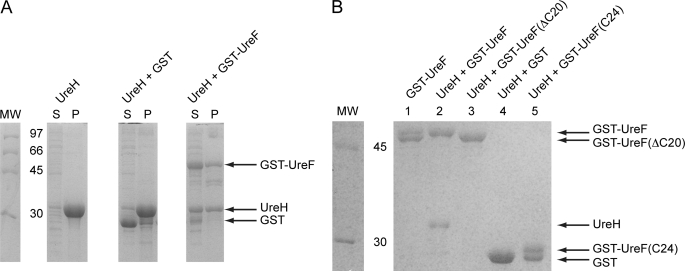
FIGURE 1.
Conserved C-terminal residues of UreF are essential for the formation of a soluble UreF-UreH complex. A, UreH was expressed alone and co-expressed with GST or GST-UreF. After cell lysis by sonication, the soluble fraction (S) and the pellet (P) were analyzed by SDS-12.5% PAGE with Coomassie Blue staining. UreH was found mainly as inclusion bodies in the pellet when expressed alone or co-expressed with GST. In contrast, a significant portion of UreH was found in the soluble fraction when co-expressed with GST-UreF. B, GST pulldown. UreH was co-expressed with GST, GST-UreF, or its variants. The soluble fraction of the bacterial lysate was loaded to a GSTrap column and eluted with 10 mm glutathione. The protein eluted was analyzed by SDS-12.5% PAGE with Coomassie Blue staining. As a control, bacteria lysate expressing GST-UreF alone was also subjected to similar procedures (lane 1). UreH was co-eluted with GST-UreF (lane 2), and no degradation of UreF was observed. However, UreH was not able to form a soluble complex with GST-UreF(ΔC20) (lane 3), GST (lane 4), and GST-UreF(C24) (lane 5). An additional band with lower molecular weight was observed for GST-UreF (lane 1) and GST-UreF(C24) (lane 5), indicating the presence of degradation.