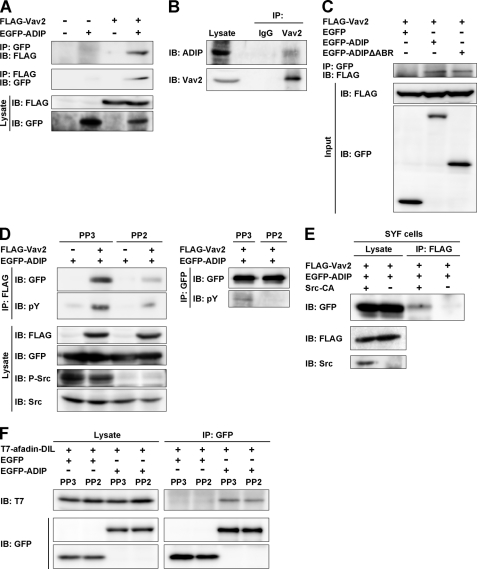
FIGURE 6.
Interaction of ADIP with Vav2 in Src phosphorylation-dependent manner. A, co-immunoprecipitation of ADIP with Vav2. Lysates of HEK293 cells transiently expressing FLAG-Vav2 and/or EGFP-ADIP were immunoprecipitated with anti-FLAG M2-agarose or anti-GFP-agarose beads followed by Western blotting with the anti-FLAG mAb or the anti-GFP pAb. B, co-immunoprecipitation of endogenous ADIP with Vav2. Lysates of HeLa cells were immunoprecipitated with the anti-Vav2 pAb or preimmune IgG as a control followed by Western blotting with the anti-Vav2 and the anti-ADIP pAbs. C, no requirement of the afadin-binding region of ADIP for the interaction of ADIP with Vav2. Lysates of HEK293 cells transiently expressing FLAG-Vav2 with EGFP, EGFP-ADIP, or EGFP-ADIPΔABR were immunoprecipitated with anti-GFP-agarose beads followed by Western blotting with the anti-FLAG mAb or the anti-GFP pAb. D, inhibition of the co-immunoprecipitation of ADIP with Vav2 by a Src inhibitor. HEK293 cells transiently expressing FLAG-Vav2 and/or EGFP-ADIP were pretreated with the Src inhibitor PP2 or the inactive analog PP3 for 1 h. Cells were lysed followed by immunoprecipitation with anti-FLAG M2-agarose or anti-GFP-agarose beads. The immunoprecipitates were subjected to Western blotting with the indicated Abs. E, no co-immunoprecipitation of ADIP with Vav2 in SYF cells. Lysates of SYF cells transiently expressing FLAG-Vav2 and EGFP-ADIP with or without the constitutively active form of Src (Src-CA) were immunoprecipitated with anti-FLAG M2-agarose beads. The immunoprecipitates were subjected to Western blotting with the indicated Abs. F, no requirement of the Src-mediated phosphorylation of ADIP for the interaction of ADIP with afadin. Lysates of HEK293 cells transiently expressing T7-afadin-DIL with EGFP or EGFP-ADIP in the presence of PP2 or PP3 were immunoprecipitated with anti-GFP-agarose beads followed by Western blotting with the anti-T7 mAb or the anti-GFP pAb. The results shown are representative of three independent experiments. IP, immunoprecipitation; IB, immunoblot; P-Src, phosphorylated Src; pY, phosphotyrosine.