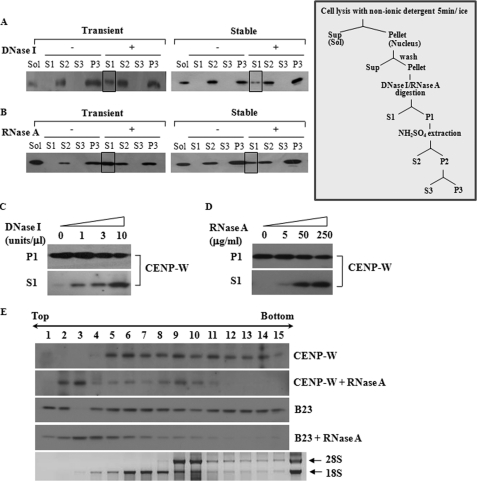
FIGURE 3.
CENP-W may form a complex with RNA as well as DNA. A, CENP-W is released by DNase I treatment. A549-FLAG-CENP-W stable cells or 293T cells transfected with FLAG-CENP-W were fractionated according to the protocols depicted below (For details, see under “Materials and Methods”). After the cells were digested DNase I (1 unit/μl), the released proteins were isolated (S1), and the remaining precipitation was further extracted using 0.25 m ammonium sulfate. The control experiment was also performed under the same conditions without nuclease. Each fraction was analyzed by Western blot analysis using anti-FLAG antibody. The boxed fraction of CENP-W corresponds to the protein released by the DNase I digestion. B, CENP-W is released by RNase A digestion. The solubilized portion of CENP-W by RNase A (100 μg/ml) digestion is highlighted as a box. C, DNase I-dependent release of CENP-W. After cells were incubated with increasing amounts of DNase I (0, 1, 3, and 10 units/μl), the supernatant was collected by centrifugation. D, RNase A-dependent release of CENP-W. After increasing amounts of RNase A (0, 5, 50, and 250 μg/ml) was added to the cells, the supernatant was separated from remaining sample. E, RNA binding assay in sucrose gradient centrifugation. The nuclear extracts isolated from HeLa-FLAG-CENP-W stable cells were incubated with or without RNase A (250 μg/ml). The resulting sample was then analyzed by 10–30% sucrose gradient sedimentation. CENP-W and B23 were identified using Western blotting with anti-FLAG and anti-B23 antibodies, respectively.