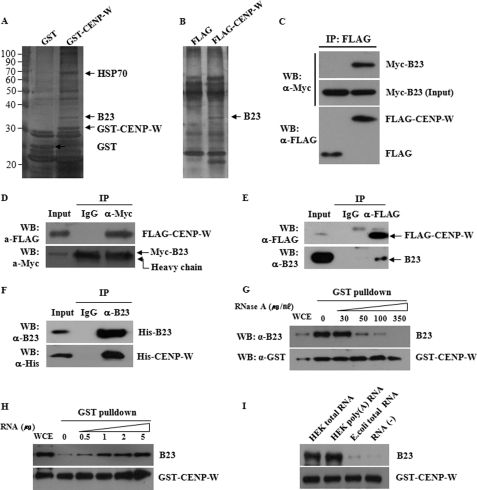
FIGURE 5.
CENP-W interacts with B23. A, affinity purification of CENP-W-interacting proteins. Nucleoli fraction obtained from cells expressing GST-CENP-W were subjected to GST pulldown analysis. Isolated proteins were then analyzed by SDS-PAGE and identified by mass spectroscopy. B, identification of CENP-W-interacting protein by using the immunoprecipitation method. C, co-immunoprecipitation between CENP-W and B23. Cell lysates transfected with Myc-B23 and FLAG-CENP-W were subjected to immunoprecipitation with anti-FLAG antibody. D, reciprocal immunoprecipitation between FLAG-CENP-W and Myc-B23 was performed using anti-Myc antibody or normal rabbit IgG. E, GST pulldown assay for endogenous B23. HeLa stable cells expressing FLAG-CENP-W were used for immunoprecipitation and endogenous B23 in complex with CENP-W was detected using anti-B23 antibody. F, in vitro binding assay. After cloning into the pET15b bacterial expression vector (Novagen), the His-tagged recombinant B23 and CENP-W was expressed in vitro using the E. coli S30 T7 protein expression system (Promega). Then, co-immunoprecipitation was carried out with anti-B23 antibody. G, GST pulldown analysis after RNase treatment. RNase was added at the indicated concentration to the lysate of H293 cells transfected GST-CENP-W, and the mixture was incubated at 25 °C for 20 min before GST pulldown analysis. Endogenous B23 was monitored using anti-B23 antibody. H, GST pulldown assay with RNA addition. After endogenous RNA is digested with 200 μg/ml RNase, RNA fractions extracted from H293 cells were added before GST pulldown assay. I, binding assay with different kinds of RNA. Three different kinds of RNA preparation (1 μg) were added to the RNase-treated sample before GST pulldown assay.