FIGURE 6.
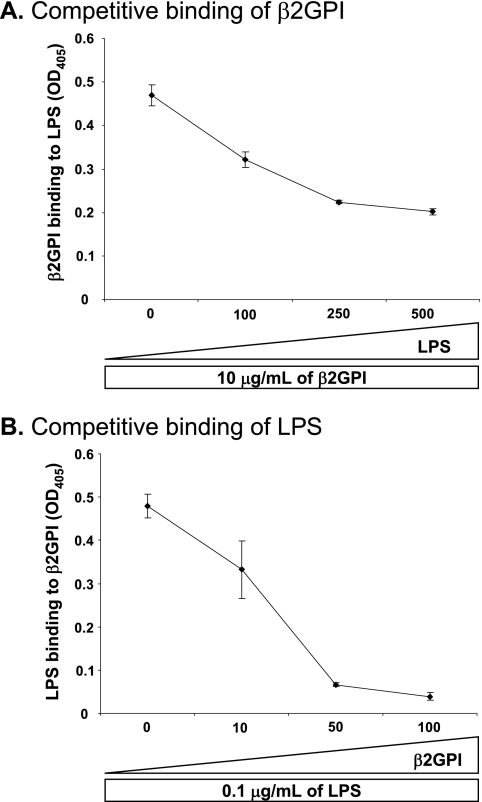
Interaction between β2GPI and LPS is specific. A, specificity of binding of soluble β2GPI to solid-phase LPS was assessed by competitive ELISA. Increasing concentrations (0, 100, 250, and 500 μg/ml) of soluble LPS were incubated with a constant concentration (10 μg/ml) of soluble β2GPI, and the mixture was then added to LPS-coated plates. Murine anti-β2GPI mAb was used to detect binding of β2GPI to plate-bound LPS. Soluble LPS inhibited the binding of β2GPI to plate-bound LPS in a dose-dependent manner. Binding is expressed as the mean A405 (OD405) ± S.D. of duplicate samples and is shown for one example representative of three independent experiments. B, specificity of binding of soluble LPS to solid-phase β2GPI was assessed by competitive ELISA. Increasing concentrations (10, 50, and 100 μg/ml) of soluble β2GPI were incubated with a constant concentration (0.1 μg/ml) of soluble LPS, and the mixture was then added to β2GPI-coated plates. Murine anti-LPS mAb was used to detect binding of LPS to β2GPI. Soluble β2GPI inhibited the binding of LPS to plate-bound β2GPI in a dose-dependent manner. Binding is expressed as the mean A405 ± S.D. of duplicate samples and is shown for one example representative of three independent experiments.