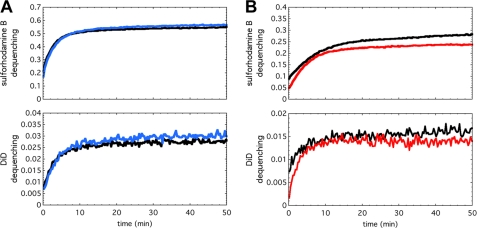
FIGURE 4.
Fusion inhibition depends on the cholesterol anchor and the specific membrane in which the antiviral peptide is anchored. (A) and (B), fluorescence-monitored fusion reactions with sulforhodamine B fluorescence (encapsulated within the liposomes), which reports liposome permeabilization (top panels), and DiD fluorescence (embedded in the viral membrane), which reports lipid mixing (bottom panels). (A), if P155–185 is not anchored to a membrane by conjugation to cholesterol, inhibition is abolished. P155–185 was incubated at 5 μm (blue traces) with 4:1 w/w DOPC:cholesterol liposomes prior to mixing with virus and acidifying to pH 5.0. The fluorescence-dequenching traces reporting on liposome content leakage (top panel) and lipid mixing (bottom panel) are essentially superimposable with 0 μm inhibitor controls (black traces). (B), P155–185-chol was incubated at 10 μm only with virus prior to mixing with DOPC:cholesterol liposomes and acidification to pH 5.0 to initiate the fusion reaction. Even with 10 μm peptide preincubated with viral particles (red traces), inhibition of liposome leakage (top panel) and lipid mixing (bottom panel) is minimal, and the fluorescence-dequenching traces closely follow the control reactions with no inhibitor present (black traces).