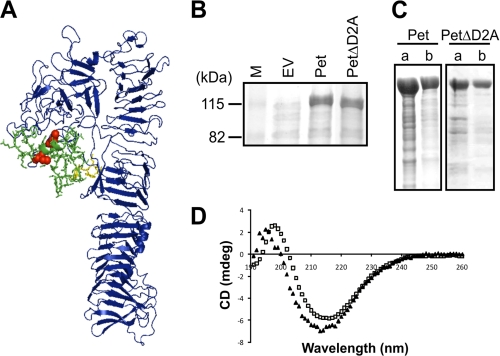
FIGURE 1.
The Cys residues and D2A are not required for secretion or folding of Pet. A, a three-dimensional model of the Pet passenger domain showing D1 and the β-helix in blue, D2A in green sticks, the Cys pair in red spheres, and the semiconserved stable platform in yellow sticks. B, SDS-PAGE analysis of TCA-precipitated culture supernatant fractions harvested after growth of TOP10 expressing empty vector (EV), Pet, and PetΔD2A. M, molecular mass markers. C, SDS-PAGE analysis of concentrated supernatants from TOP10 expressing Pet and PetΔD2A before (lane a) and after (lane b) gel filtration chromatography. D, far-UV CD spectra of Pet (open squares) and PetΔD2A (closed triangles) in millidegrees (mdeg) showing maxima and minima at 199 and 217 nm and 196 and 214 nm, respectively.