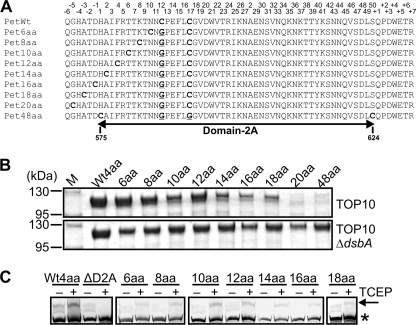
FIGURE 2.
Large disulfide-bonded regions abolish secretion of Pet. A, alignment of D2A from Pet derivatives showing site-directed mutations that sequentially increased the distance between the endogenous Cys pair in D2A (PetWt) until OM translocation and secretion of the Pet passenger domain were stalled and abolished, respectively. Endogenous and exogenous Cys pairs are in bold font, and residues mutated to Gly are in bold font and underlined. Although D2A resides within residues 575–624 of the full-length protein, the numbers on top correspond to the position of each residue within D2A from His-1 to Ser-50. B, SDS-PAGE analysis of TCA-precipitated culture supernatant fractions harvested after growth of TOP10 (top panel) and TOP10 ΔdsbA (bottom panel) expressing wild-type Pet (Wt4aa) and Pet6aa to Pet48aa. M, molecular mass markers. C, mPEG-Mal labeling of wild-type Pet (Wt4aa), PetΔD2A, and Pet6aa to Pet48aa in the presence and absence of TCEP. Supernatants were harvested and concentrated after growth of TOP10 cells expressing wild-type Pet, PetΔD2A, and Pet6aa to Pet48aa. Samples were resolved on a gradient 4–20% Tris-HEPES-SDS-PAGE gel, and Pet was localized by Western immunoblotting using anti-Pet passenger antibody. The arrow indicates labeled/pegylated Pet, and the asterisk shows unlabeled/unpegylated Pet.