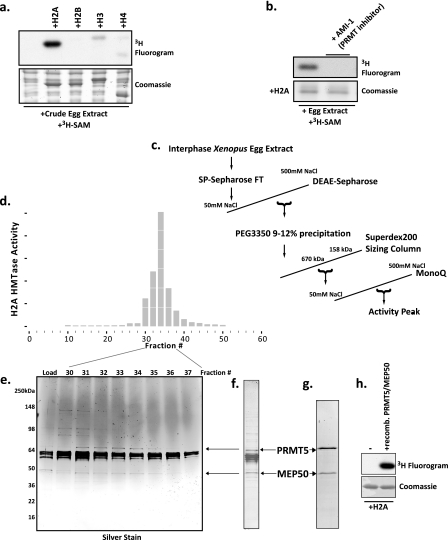
FIGURE 1.
Purification and identification of an abundant histone H2A and H4 arginine 3 methyltransferase in Xenopus laevis egg extract as a complex of Prmt5 and Mep50. a, Xenopus egg extract was incubated with recombinant histones H2A, H2B, H3, and H4 and with the methyl donor [3H]AdoMet ([3H]SAM). Shown are a Coomassie-stained SDS gel (bottom) and a fluorogram (top). b, recombinant H2A was incubated with egg extract and [3H]AdoMet in the presence or absence of 50 μm AMI-1. Bottom, Coomassie-stained SDS gel; top, corresponding fluorogram. c, chromatography scheme for the purification of the H2A methyltransferase activity. d, full-length histone H2A methyltransferase activity across the fractions of the MonoQ column (final column in the purification) performed as a P81 filter binding assay; the HMTase activity histogram showed peak activity in fractions 32–34. e, silver-stained gel of the peak activity fractions. f, Coomassie-stained SDS gel of the pooled peak activity fractions 32–34. Stained bands were identified by mass spectrometry (supplemental Fig. S1), and the location of the arginine methyltransferase Prmt5 and its known cofactor Mep50 are shown with arrows on the gel and in the silver-stained gel. g, Coomassie-stained SDS gel of recombinant Prmt5 and Mep50 produced in baculovirus-infected Sf9 cells. h, methyltransferase assay demonstrating that the recombinant Prmt5-Mep50 complex methylates recombinant H2A (fluorogram, top); lane 1 did not contain enzyme, whereas lane 2 did contain enzyme, and both lanes contained H2A.