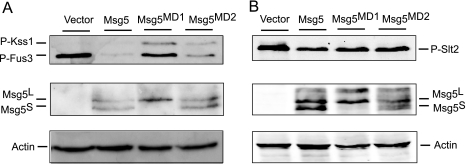
FIGURE 4.
Effect of mutation of the putative Msg5 docking domains on MAPK phosphorylation. A, Western blot analysis of cell extracts from the DD1-2D (msg5Δ) strain transformed with the empty vector YCplac22, YCplac22MSG5m, YCplac22MSG5MD1m, or YCplac22MSG5MD2m expressing Msg5–6Myc, Msg5MD1-6Myc, or Msg5MD2-6Myc, respectively. Cells were grown to mid-log phase in YPD medium at 24 °C and then treated with 50 nm α-factor for 10 min. B, Western blot analysis of cell extracts from the same strains as in A, after treatment with 30 μg/ml Congo red for 2 h. Analyses were performed using anti-phospho-p44/p42 MAPK (Thr-202/Tyr-204) antibody for immunodetection of phosphorylated Fus3, Kss1, and Slt2 (top), anti-Myc for detecting the two Msg5 isoforms Msg5S and Msg5L (23) (middle), and anti-actin antibodies for actin detection as loading control (bottom). Reproducible results were obtained in different experiments, and selected images correspond to representative blots.