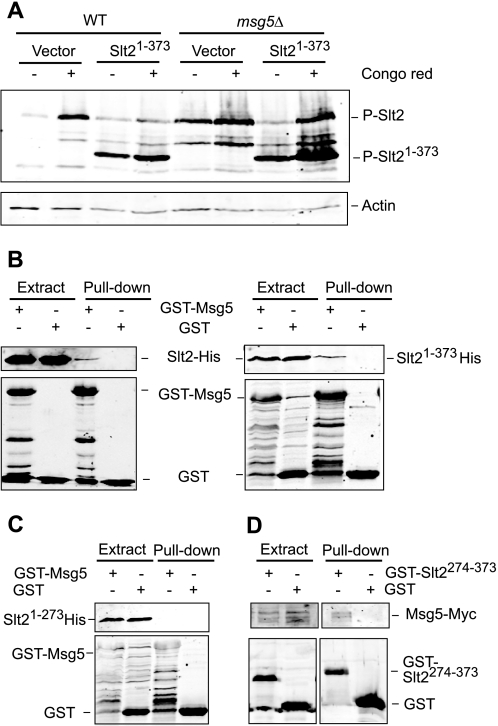
FIGURE 6.
Mapping of the Slt2 region that mediates binding to Msg5. A, Western blot analysis of cell extracts from the BY4741 strain (WT) and the isogenic mutant strain Y07373 (msg5Δ) transformed with the vector YCpLac111 or plasmid YCplac111-Slt2(1–373). Cells were grown to mid-log phase in YPD medium at 24 °C (−) and then treated with 30 μg/ml Congo red (CR) for 2 h as indicated. Immunodetection was performed with anti-phospho-p42/44 MAPK (top) and anti-actin (bottom) antibodies as loading control. B and C, Western blot analysis of the in vitro co-purification of recombinant Msg5 with Slt2 and different truncated Slt2 versions. E. coli extracts containing GST or GST-Msg5 were incubated with E. coli extracts containing Slt2-His, Slt2(1–373)-His (B), or Slt2(1–274)-His (C) and glutathione-Sepharose to pull down GST-complexes. Immunodetection was performed using anti-poly-His (top) and anti-GST (bottom) antibodies. D, Western blot analysis of co-purification of Msg5 with Slt2(274–373) fragment. Yeast extracts of the Msg5–6myc-tagged strain YMF1 transformed with the indicated plasmid, pEG(KG) (GST) or pEG(KG)-SLT2(274–373) (GST-SLT2274–373), were incubated with glutathione-Sepharose to pull down GST complexes. Immunodetection was performed with anti-Myc (top) and anti-GST antibodies (bottom). Reproducible results were obtained in different experiments, and selected images correspond to representative blots.