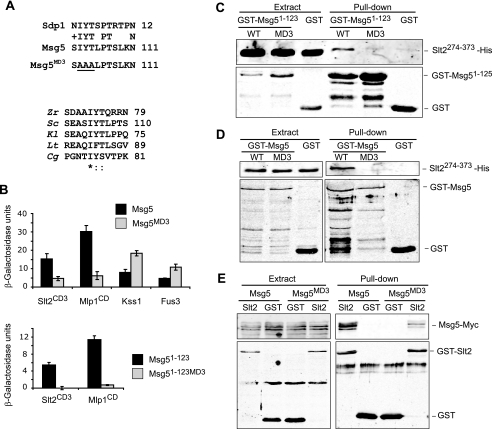
FIGURE 8.
The MD3 motif mediates binding of Msg5 to Slt2 and Mlp1. A, top, sequence comparison by BLAST analysis between Msg5 and Sdp1 revealed a similar region in the N-terminal part of these two proteins. The area corresponding to the putative Msg5 motif MD3 is shown here. Amino acidic changes introduced in the mutated version Msg5MD3 are underlined. Bottom, multiple alignment of the Msg5 amino acid sequence stretch bearing the MD3 motif with several Msg5 orthologs from different fungi. Alignment was performed by ClustalW (available on the World Wide Web). Identical residues are indicated by asterisks. Dots mark similar residues. Zr, Zygosaccharomyces rouxii; Sc, S. cerevisiae; Kl, Kluyveromyces lactis; Lt, Lachancea thermotolerans; Cg, Candida glabrata. B, semiquantitative analysis of the two-hybrid interaction of diploid cells containing plasmids that encode the indicated proteins. Before mating, pGBKT7, pGBKT7-Msg5, pGBKT7-Msg5MD3, pGBKT7-Msg5(1–123), or pGBKT7-Msg5(1–123)MD3 were transformed into PJ69-4A cells, and pGADT7, pGADT7-Kss1, pGADT7-Fus3, pGADT7-Slt2CD3, or pGADT7-Mlp1CD were transformed into PJ69-4α cells. Two-hybrid assays were performed as indicated in Fig. 1B. C, Western blot analysis of the in vitro co-purification of recombinant GST-Msg5(1–123) (WT) and GST-Msg5(1–123)MD3 (MD3) with Slt2(274–373)-His. E. coli extracts containing GST fusions were incubated with E. coli extracts containing Slt2(274–373)-His and glutathione-Sepharose to pull down GST complexes. Immunodetection was performed using anti-poly-His (top) and anti-GST (bottom) antibodies. D, Western blot analysis of the in vitro co-purification of recombinant GST-Msg5 (WT) and GST-Msg5MD3 (MD3) with Slt2(274–373)-His. E. coli extracts containing GST fusions were incubated with E. coli extracts containing Slt2(274–373)-His and glutathione-Sepharose to pull down GST complexes. Immunodetection was performed as in C. E, Western blot analysis of Msg5–6Myc pulled down by GST-Slt2. Transformants of the yeast DD1-2D strain with YCplac22MSG5m and pEG(KG) (GST) or pEG(KG)-SLT2 (Slt2) were grown to mid-log phase in selective medium at 24 °C, cells were collected, cell extracts were prepared, and GST-Slt2 complexes obtained by purification with glutathione-Sepharose were analyzed by immunoblotting with anti-Myc (top) and anti-GST (bottom) antibodies. In all cases, reproducible results were obtained in different experiments, and selected images correspond to representative blots.