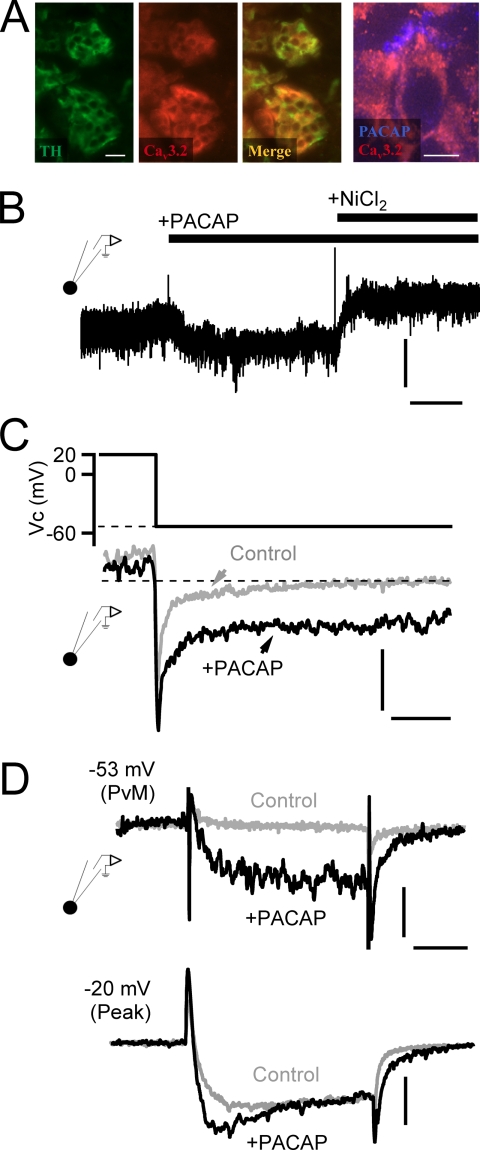
FIGURE 2.
PACAP facilitates nickel-sensitive current at negative potentials. A, immunostaining for tyrosine hydroxylase (TH; green panel), a marker for catecholamine-secreting adrenal chromaffin cells, and Cav3.2 T-type calcium channels (red panel) are shown. The merge overlay (“Merge”) indicates that both signals are present in the same cells of the adrenal medulla (scale bar, 50 μm). The panel to the right shows staining for PACAP and Cav3.2 at a higher resolution (scale bar, 10 μm) and shows a peripheral staining for PACAP surrounding the Cav3.2-positive chromaffin cell. B, a chromaffin cell was held at −53-mV command potential to match PVm, and the membrane current was measured. Puffing of exogenous PACAP (1 μm) elicited a sustained inward current. Vertical scale bar, 6 pA/picofarad; horizontal scale bar, 30 s. The PACAP-evoked current was blocked by the additional perfusion with 50 μm NiCl2. C, a protocol was designed to assess PACAP-dependent effects on Ca2+ tail currents. Representative records are provided, demonstrating that a depolarization to 20 mV and return to PVm resulted in a specific enhancement of a non-inactivating window current in PACAP-treated cells. Vertical scale bar, 6 pA/picofarad; horizontal scale bar, 10 ms. D, voltage clamp depolarization from −80-mV holding potential to PVm (−53 mV) elicits both a specific augmentation of inward current and a slower deactivating tail current specifically after stimulation with exogenous PACAP (10 μm). Depolarization to −20 mV (“Peak”) to maximally activate all voltage-gated calcium channels elicits a specific emergence of a rapidly activating component as well as a slowly deactivating tail current after PACAP stimulation. Horizontal scale bar, 15 ms; vertical scale bar: −53 mV, 4 pA/picofarad; −20 mV, 5 pA/picofarad.