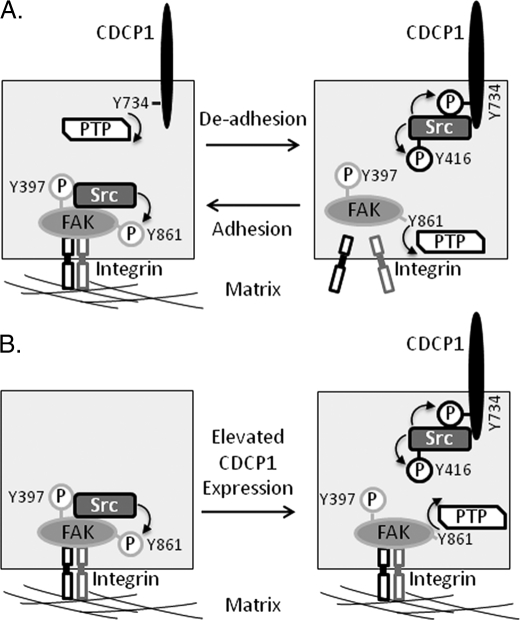
FIGURE 10.
SFK switching between FAK-Tyr-861 and CDCP1-Tyr-734. A, in adherent cells Src binds to p-FAK-Tyr-397 and phosphorylates several other FAK tyrosines including Tyr-861 (45). We and others (20, 23, 29) have shown that CDCP1-Tyr-734 is rapidly dephosphorylated during cell adhesion, but the protein-tyrosine phosphatase (PTP) that mediates this dephosphorylation is not yet known. Src phosphorylation of CDCP1-Tyr-734 also results in increased phosphorylation of Src-Tyr-416. In de-adhered cells our data indicate that Src switches to phosphorylation of CDCP1-Tyr-734, and we have shown that FAK-Tyr-861 is rapidly dephosphorylated in this setting. The PTP that mediates FAK-Tyr-861 dephosphorylation is also not known. B, in cells with elevated expression of CDCP1, Src phosphorylates CDCP1-Tyr-734, which is accompanied by dephosphorylation of FAK-Tyr-861. In HeLa cells stably expressing CDCP1, phosphorylation of FAK-Tyr-397 is maintained, indicating that the kinase activity of FAK, and its role in focal adhesion is retained.