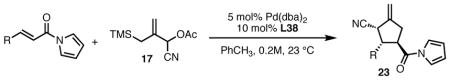
Table 6.
Substrate Scope of the TMM Reaction of Acylpyrroles with Nitrile Donor 17.
 | |||||
|---|---|---|---|---|---|
| entry | substrate | product | yield (%) | dr | ee (%) |
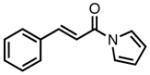
| 1b |
|
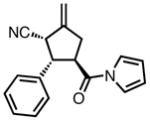 23a |
87 | >20:1 | 92 |
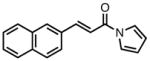
| 2 |
|
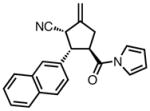 23b |
84 | >20:1 | 94 |
| 3 |
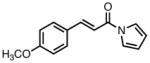
|
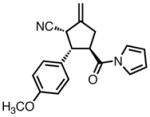 23c |
80 | >20:1 | 94 |
| 4 |
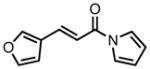
|
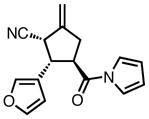 23d |
98 | >20:1 | 95 |
| 5 |
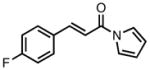
|
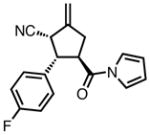 23e |
96 | >20:1 | 94 |
| 6 |
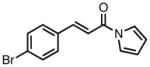
|
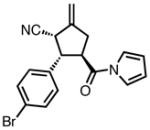 23f |
100 | >20:1 | 95 |
| 7 |
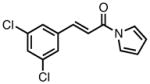
|
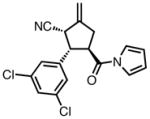 23g |
90 | > 20:1 | 97 |
| 8 |
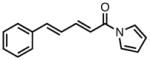
|
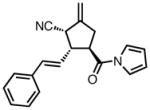 23h |
63 | > 20:1 | 95 |
| 9 |
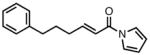
|
 23i |
82 | > 20:1 | 94 |
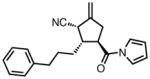
| 10 |
|
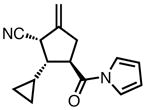 23j |
84 | > 20:1 | 94 |
All reactions were performed at 0.2M in toluene with 5% Pd(dba)2, 10% ligand L38, 1.5 equiv 17 and stirred for 3h; yields were combined isolated yields; ee’s were determined by HPLC with a chiral stationary phase column.
Reaction performed at 50 °C.
