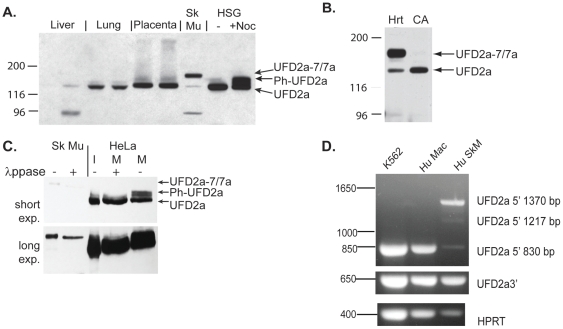
Figure 1. A larger isoform of UFD2a is specifically expressed in striated muscle.
A). Protein lysates were prepared from normal human tissues and human salivary gland cells (HSG) that were untreated (-) or arrested in mitosis (+Noc) and subjected to SDS PAGE. After transfer to nitrocellulose, samples were analyzed by immunoblotting using a rabbit polyclonal antibody recognizing UFD2a. A 97-kDa band visible in many muscle lysates and some other tissues represented cross-reactivity to phosphorylase b (not shown). B). UFD2a-7/7a expression in cardiac muscle (Hrt) but not coronary artery (CA) from an explanted human heart (3 patient samples were analyzed; 1 is shown here). C). Human skeletal muscle (Sk Mu) lysates and lysates made from human HeLa cells arrested in mitosis (M) with nocodazole were treated with λprotein phosphatase (λppase). Interphase (I) and untreated mitotic (M) HeLa cells are shown for comparison; phos refers to the phosphorylated form of UFD2a. Top and bottom panels represent different exposures of the same Western blot to account for the difference in the UFD2a levels in total protein from cell cultures compared to human tissue. D).Total RNA from human skeletal muscle (Hu SkM), K562 human erythroleukemia cells, and primary human monocyte-derived macrophages (Hu Mac) were used as templates for cDNA reactions. RT-PCR analyses were performed using primers recognizing a portion from the 5′-end of UFD2a (upper panel), the 3′-end of UFD2a (middle), or from the housekeeping gene HPRT (lower). The 5′ UFD2a primers amplified the expected 830-bp fragment from other tissues and the novel 1217-bp and 1370-bp fragments in muscle.