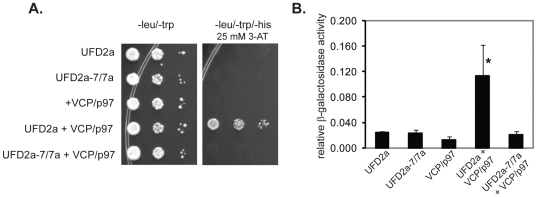
Figure 7. The muscle-specific UFD2a-7/7a isoform does not interact with VCP/p97.
A). Yeast cells transformed with bait plasmids pDEST32-UFD2a, pDEST32-UFD2a-7/7a, or empty pDEST32 and prey plasmids pDEST22-VCP/p97 or empty pDEST22 were grown in liquid double dropout medium (-leu, -trp) to saturation and diluted to an OD600 of 1. Serial dilutions of these cell cultures were prepared in 96-well plates and transferred to triple-dropout agar plates (-leu, -trp, -his) supplemented with 25 mM 3-AT. B). Isolated colonies of yeast cells transformed (as in panel A) were grown to saturation, diluted to OD600 of 0.3, and grown to log phase (OD600 = 0.8-1.5). Cells were disrupted with glass beads in Z buffer; ONPG substrate and β-mercaptoethanol were added to the resulting supernatants. After incubation at 37°C, supernatants were transferred to 96-well plates and read at 420 nm. B). Relative β-galactosidase activity is calculated from 6 independent experiments; error bars indicate standard error. The average activity of UFD2a+VCP/p97 was significantly higher than that of the other transformants (*p = 0.02). The activity of UFD2a-7/7a+VCP/p97 did not differ from that of the negative controls.