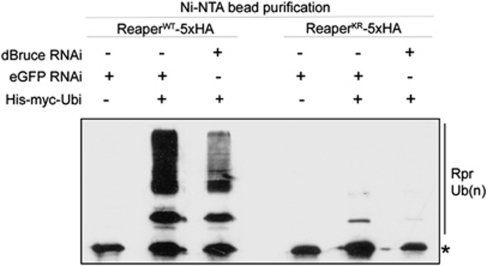
Figure 5.
dBruce dependent ubiquitination of wild-type and lysine-deficient Reaper proteins. In order to purify ubiquitinated Reaper proteins from cultured cells, S2R+ cells were transfected with Reaper-5 × HA and His-tagged ubiquitin expression plasmids. Cells were treated with a proteasome inhibitor before his-ubiquitin-tagged proteins were purified from these extracts using Ni-NTA resin. The Reaper protein was detected through western blot with anti-HA antibody. The asterisk indicates the band corresponding to unmodified Reaper protein that was retained through unspecific matrix interaction. Higher molecular weight bands of Reaper-WT-5 × HA were detected only when His-ubiquitin was co-transfected (lanes 2, 3, 5 and 6), indicative of ubiquitin conjugation. His-ubiquitin dependent higher molecular weight Reaper-KR was also detected, although at a lower extent (lane 5). Knockdown of dBruce by RNAi decreases the abundance of the ubiquitinated species for both Reaper-WT and in Reaper-KR, supporting the idea that dBruce ubiquitinates Reaper on non-lysine residues