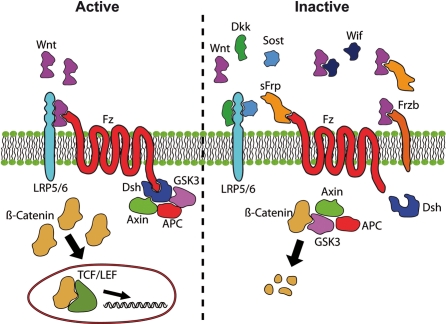
Figure 1.
Canonical Wnt signaling. In the active state, Wnt ligands (Wnt) form a complex with the receptors low-density lipoprotein receptor-related protein 5 or 6 (LRP5/6) and Frizzled (Fz). Disheveled (Dsh) is then able to bind to Fz. Dsh forms a complex with glycogen synthase kinase 3ß (GSK3ß), Axin, and adenomatous polyposis coli (APC). This protects ß-catenin from proteasomal degradation so that ß-Catenin can accumulate in the cytosol and translocate to the nucleus. In the nucleus it interacts with the T-cell factor/lymphoid enhancer factor (TCF/LEF) family of transcription factors, leading to gene transcription. Inhibitors of this system prevent the formation of the Wnt-Fz-LRP5/6 complex, inactivating Wnt signaling. This leads to GSK3ß-mediated phosphorylation of ß-catenin, causing it to be degraded. A variety of extracellular inhibitors serve as inactivators. Dickkopf (Dkk) and sclerostin (Sost) bind to LRP5/6, preventing Wnt binding. Secreted Frizzled-related protein (sFrp) has similarities with Frizzled, and can bind either to the Wnt ligand or to the Fz receptor itself. Frizzled-related protein (Frzb) acts as a decoy receptor for Wnt ligands. Wnt-inhibitory factor (Wif) also binds directly to the Wnt ligand (Komatsu and Warden 2010, MacDonald 2009, Macsai 2008).