Fig 2.
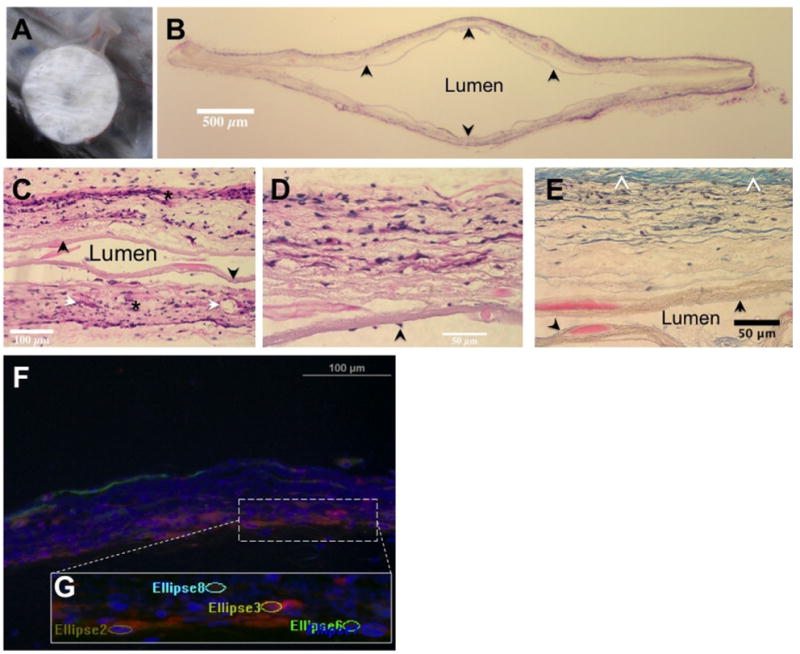
(A) Subcutaneously implanted immunoisolation device at time of explant. Note the vascularity and lack of large fibrotic response. (B) (H&E) stained transverse section of a device (original magnification, 1×). The central lumen is cell-free, the outer layer shows significant cellular infiltration. The inner layer (arrow) is intact through the cross-section. (C) H&E stained image of the central luminal area clearly shows tissue integration of outer layers (*) on either side while the intact inner layers (arrow) serve as a barrier to cells moving across (original magnification, 20×). Also identified are some vascular elements (white arrows). (D) H&E section focused on a single wall of the device reinforces the tissue integrative and cellular barrier natures of the outer and inner layers, respectively (original magnification, 40×). Scattered cells on the luminal aspect (arrow) without apparent breach of inner layer may suggest migration of cells from an area of loss of integrity. (E) Section of the device wall stained with Masson's trichrome (original magnification, 40×). Note the relatively small amount of collagenous tissue capsule (white arrowheads). The inner (black arrow) and outer membranes are clearly demarcated, confirming the cellular penetration of only the outer layer of the device while the lumen is cell- free. (F) Composite image of immunostained sections showing vascular structures, smooth muscle cells, and cell nuclei. (G) The ellipsoid regions counted as vascular elements overlaid on a magnified area of the image.
