Abstract
Objective:
We describe the systematic approach to incidental findings (IFs) used at the Mind Research Network (MRN) where all MRI scans receive neuroradiologist interpretation and participants are provided results.
Methods:
From 2004 to 2011, 8,545 MRI scans were acquired by 45 researchers. As mandated by MRN′s external institutional review board, all structural sequences were evaluated by a clinical neuroradiologist who generated a report that included recommendations for referral if indicated. Investigators received a copy of their participants' reports, which were also mailed to participants unless they specifically declined. To better understand the impact of the radiology review process, a financial analysis was completed in addition to a follow-up phone survey to characterize participant perceptions regarding receiving their MRI scan results.
Results:
The radiologist identified IFs in 34% of the 4,447 participants. Of those with IFs (n = 1,518), the radiologist recommended urgent or immediate referral for 2.5% and routine referral for 17%. For 80.5%, no referral was recommended. Estimated annual cost for this approach including support for the neuroradiologist, medical director, and ancillary staff is approximately $60,000 or $24/scan. The results of the retrospective phone survey showed that 92% of participants appreciated receiving their MRI report, and the majority stated it increased their likelihood of volunteering for future studies.
Conclusions:
Addressing IFs in a cost-effective and consistent manner is possible by adopting a policy that provides neuroradiology interpretation and offers participant assistance with clinical follow-up when necessary. Our experience suggests that an ethical, institution-wide approach to IFs can be implemented with minimal investigator burden.
An incidental finding (IF) is defined as having “… potential health or reproductive importance and is discovered in the course of conducting research but is beyond the aims of the study.”1 Despite the prevalence of IFs in neuroimaging research,2–5 there is a surprising lack of consensus regarding an approach to management. Radiology reviews of research scans are perceived as time-consuming and expensive and potentially exposing institutions to risk.6–8 In addition, there are concerns that receiving a radiology report may cause unnecessary anxiety among research participants or unfairly burden those who are uninsured.9 Conversely, neuroimaging IFs may have medical importance, and a large majority of research participants prefer to be informed of their radiology review.10 In 2004, the University of New Mexico Health Sciences Center institutional review board (IRB) mandated that all research MRI scans be read by a neuroradiologist to identify IFs. The Mind Research Network (MRN) created a systematic approach to radiology reviews by contracting with a board-certified neuroradiologist to provide MRI readings and by developing Health Insurance Portability and Accountability Act–compliant information systems to provide investigators and participants copies of radiology reports. The current study represents a comprehensive examination of this approach, including a summary of IFs, costs, and participants' perceptions.
METHODS
Our approach to radiology review is summarized from 45 principal investigators (PI) who conducted 134 studies at MRN from August 2004 to February 2011. Areas of investigation included neurologic and psychiatric disorders with an emphasis on addictions, psychopathy, aging, schizophrenia, and brain injury. A total of 8,545 brain MRI scans were collected from 4,447 participants with diagnosed illnesses and healthy control subjects. The mean age of participants was 30 years; 62% were male (age range 0.3–89 years), and 38% were female (age range 0.3–90 years).
Conceptualization and implementation.
Our model began with a mandate from the University of New Mexico Health Sciences Center IRB to have all research MRI scans evaluated for IFs. Although all MRIs were evaluated for IFs, some PIs provided the radiology report in all cases, whereas others only informed participants when a referral was recommended. This approach raised a concern that participants were not being treated equitably across all studies and resulted in confusion among participants. Therefore, MRN developed a centralized, standardized approach to IFs that was developed with PIs and implemented over several months. After the process was initiated, modifications were made to improve efficiency in uploading and reviewing the scans and in distributing the results to investigators and participants.
Standard protocol approvals, registrations, and patient consents.
All participants were enrolled in IRB-approved protocols, provided informed consent, and completed a standardized MRI safety screening form. Exclusion criteria were determined by the individual study protocols. A separate protocol, which included a waiver of documentation of consent for the phone survey, was approved for the participant perception study.
All scans were performed at MRN on 1 of 3 MRI scanners. MRI sequences varied depending on study protocols and included at least one anatomic scan; complete clinical scanning was not part of any study. Using OsiriX (www.osirix-viewer.com/) and an in-house–developed neuroinformatics software system (Medical Imaging Computer Information System [MICIS]),11 reviews were completed and securely e-mailed to the PI using a code in lieu of participants' names to ensure confidentiality. A copy of the radiology report was mailed to participants with a cover letter thanking them for participating, providing them contact information for questions, and reminding them the scan was for research purposes only (appendix e-1 on the Neurology® Web site at www.neurology.org). Exceptions to mailing reports occurred when 1) the participant requested not to receive the review, 2) the PI was a physician and preferred to hand-deliver reports to his or her participants, or 3) the participant was incarcerated, in which case the report was provided to the prison medical director according to prison protocols. Total time required to complete this process varied from 1 to 4 weeks depending on the radiologist's schedule and the time required to transfer mobile MRI scans to the main facility for review.
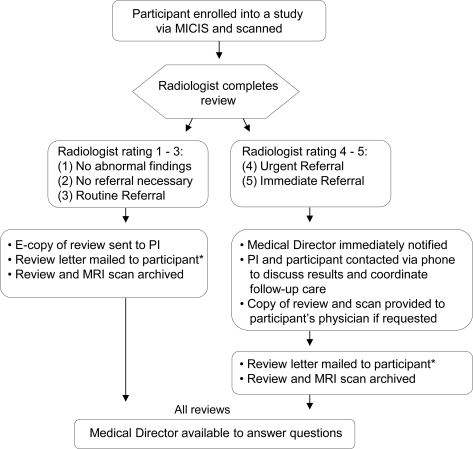
The radiology report used a 5-point Likert rating scale to identify and classify IFs as follows (modified from Katzman et al.5): 1) no abnormal findings; 2) no referral necessary; 3) routine referral; 4) urgent referral; or 5) immediate referral. The medical director was immediately notified by the neuroradiologist when a level 4 or 5 finding was discovered. The study PI and the participant (or guardian) were then contacted by the medical director to assist with coordinating clinical care (figure).
Figure. Flow chart of radiology review process.
Flow chart of Mind Research Network's radiology review process from study enrollment to receipt of radiology report. *Letters are mailed directly to participant. Exceptions include minor participants (mailed to guardian), incarcerated participants (provided to prison medical director), and PI who is a clinician and elects to provide review letter in person. MICIS = Medical Imaging Computer Information System (Participant Database).
The rate of IFs was calculated using the highest rating per participant when multiple scans were reviewed and the most recent scan if more than one scan had the same rating. For patient populations, expected findings (e.g., white matter lesions in a participant with known multiple sclerosis) were excluded from IF calculations.
To quantify the financial burden of the radiologic review process, an analysis was completed to calculate the cost for the past 2 years. The analysis included average weekly labor for all personnel (neuroradiologist, medical director, research operations, neuroinformatics engineer, information technology, and administrative staff), equipment, and supplies required to review scans and disseminate results.
In an effort to characterize the impact of this approach, we conducted a phone survey of 50 participants who had a single radiology review within the prior 2 years. Adult, nonincarcerated participants were randomly selected to produce a sample of approximately equal gender and IF rating distribution representative of the complete MRN IF database. In a scripted phone survey, 9 questions were asked regarding participant perceptions and the impact of receiving their radiology review.
RESULTS
IF frequency.
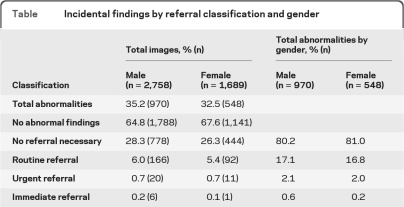
Consistent with prior reports,2,3,5 the radiologist identified IFs in 34% of the 4,447 participants. Of those with IFs (n = 1,518), the radiologist recommended urgent or immediate referral for 2.5% and routine referral for 17%; 80.5% required no referral. The incidence of IFs was similar for incarcerated and nonincarcerated populations. The table shows IFs by gender.
Table.
Incidental findings by referral classification and gender
Financial costs.
The financial analysis included all labor, equipment, and supplies necessary to review and disclose MRI scan results. In addition to the ongoing costs, there was an initial investment to develop and test the addition of a radiology review feature in the existing MICIS database.11 The annual cost for all personnel, support, and supplies is approximately $60,000, less than $24/scan based on an average of 2,500 reviews per year. The majority of the total cost (94%) is for labor, and the remainder is for computer equipment, monitors, and mailing supplies. The cost of the radiology review is included in the hourly MRI scanner rate.
Participant perception.
Of participants surveyed, 72% expected to be told of their scan findings, 10% of participants sought further medical evaluation based on their MRI report, 92% appreciated receiving the information, and 58% said they are more likely to participate in future studies because they received the radiology review.
DISCUSSION
This report summarizes experience with a mandated radiology review of research MRIs and the system implemented to ensure equitable treatment across all studies at a large neuroimaging center. Because of the volume of scans performed, systems were developed to automate the review process as much as possible and distribute costs over multiple investigators, reducing the per-scan cost. Participant response to this approach was positive based on our retrospective phone survey. Thus, our experience suggests it is possible to provide a consistent review for IFs in neuroimaging research that is cost-effective, minimizes investigator burden, and addresses key ethical principles such as beneficence.
There is currently no consistent method for addressing IFs in neuroimaging research. Published approaches range from suggesting no reviews,7 to selective reviews (at the discretion of the PI),12 to full clinical reviews for all research scans.13 Our approach is different. Mandating radiology review for all participants not only addresses the ethical principle of justice (treating participants across all research protocols at MRN equally) but also reduces the cost considerably. In addition, participants benefit by having a review of an MRI, which may identify findings of clinical importance now or in the future. Finally, a key aspect of this approach to IFs is that all participants receive a copy of their radiology report, regardless of findings, unless the participant declines to receive this information. The decision to receive a copy of the radiology report is thus left to the individual participants, not to the researchers, respecting individual autonomy. Clearly, as noted by our survey and in a prior report,10 an overwhelming majority of research participants are interested in their MRI findings. This comprehensive approach to IFs is consistent with the cornerstone principles of the Belmont Report (respect for persons, beneficence, and justice), which provide the ethical foundation for research in the United States.14
There are several shortcomings to this study. We do not explore the ethical issues involved if a participant were to decline an MRI report that contained a serious IF. In addition, although our phone survey addressed general questions regarding participant perceptions, it did not probe issues of health literacy or how perceptions might change over time, nor did we survey other important stakeholders such as investigators or IRB members. Finally, although we provide an accurate ongoing cost analysis of our system, it is more difficult to account for all initial developmental costs such as testing and system modifications.
More work is needed to clarify the optimal approach to IFs in neuroimaging research, and the debate regarding methods of fulfilling obligations to research participants is far from over. However, this model demonstrates there is a practical option that satisfies the needs of most investigators, promotes ethical conduct of research, remains cost-effective, and could be adopted by other centers.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the following contributors for use of their imaging data for this manuscript: Christopher Abbott, MD; Steven Adelsheim, MD; William Apfeldorf, MD; Angela Bryan, PhD; Juan Bustillo, MD; Jose Canive, MD; Vince Clark, PhD; Eric Claus, PhD; Francesca Filbey, PhD; Corey Ford, MD; Chuck Gasparovic, PhD; Glen Graham, MD; Faith Hanlon, PhD; Dina Hill, PhD; Kent Hutchison, PhD; Rex Jung, PhD; Patricia Kapsner, MD; Piyadasa Kodituwakku, MD; Yuku Komesu, MD; Jim Kroger, PhD; Benjamin Ladd; John Lauriello, MD; Barbara McCrady, PhD; Leslie Morrison, MD; Paul Mullins, PhD; Stephan Posse, PhD; Carlos Roldan, MD; Gary Rosenberg, MD; Joseph Sadek, PhD; Dan Savage, PhD; Matthew Shane, PhD; Bruce Smith, PhD; Claudia Tesche, PhD; Ursina Teuscher, PhD; Robert Thoma, PhD; Gerardo Villarreal, MD; Peter Volegov, PhD; Michael Weisend, PhD; and Ronald Yeo, PhD.
GLOSSARY
- IF
incidental finding
- IRB
institutional review board
- MICIS
Medical Imaging Computer Information System
- MRN
Mind Research Network
- PI
principal investigator
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
J.M. Shoemaker: drafting/revising the manuscript for content, study concept and design, analysis and interpretation of data, acquisition of data, study coordination. Dr. Holdsworth: drafting/revising the manuscript. Dr. Aine: drafting/revising the manuscript for content, acquisition of data. Dr. Calhoun: drafting/revising the manuscript for content, acquisition of data, study coordination. R. de La Garza: study concept. Dr. Feldstein Ewing: drafting/revising the manuscript for content, acquisition of data. Dr. Hayek: drafting/revising the manuscript for content, study concept. Dr. Mayer: drafting/revising the manuscript for content, analysis and interpretation of data, acquisition of data. Dr. Kiehl: drafting/revising the manuscript for content, acquisition of data. L.E. Petree: drafting/revising the manuscript for content, study design, acquisition of data. Dr. Sanjuan: drafting/revising the manuscript for content, study concept, acquisition of data, study coordination. A. Scott: drafting/revising the manuscript for content, contribution of vital tools, acquisition of data, implementation of process in software applications. Dr. Stephen: drafting/revising the manuscript for content, acquisition of data. Dr. Phillips: drafting/revising the manuscript for content, study concept and design, analysis and interpretation of data, study coordination, acquisition of data.
STUDY FUNDING
This study was not industry-sponsored; however, data presented from industry-sponsored studies were included in the analysis. This material is based on work supported by NIH, NCCR, the Department of Energy, Defense Advanced Research Projects Agency, Delle Foundation, John Templeton Foundation, University of New Mexico, CTSC, National Multiple Sclerosis Society, Office of Naval Research, Sandia University Research Program, and Swiss NSF under award numbers DE-FG02-08ER64581, CTSC001-1, 1-KL2-RR31976-1, 1-UL1-RR031977, 1-R01-AG029495-03, 1-UL1-RR031977, 1-R21-MH080141-02, 1-R01-AG020302-05, R01-AA017390, 5-P20-RR021938, DE-FG02-08ER64581, 1-R03-DA027892-01, R01-MH072681, R01-MH070539, 1-R01-MH085010-01A1, R01-MH071896, R01-DA020870, 1-R01-DA026505-01A1, 5-P20-AA017068, DE-FG02-08ER64581, 1-R03-DA022435-01A1, R24-HD050836, R21-NS064464-01A1, 5-R21-HD041237-02, 5-M01-RR00997, 5-M01-RR000997-35, 1-R01-HD059856-01A2, 1-R01-EB002618-01, 1-R21-DA027149-A1, 1-R21-DA029464, 1-R41-NS062474-01, DE-FG02-99ER62764, 1-R01-DA14178-01, R01-HL04722-A5, 3-R01-NS052305, 1-R21-DA027149-01, P20-AA017068, 5-U24-AA014811-06, 1-R21-DA025135, PA001-113097, NBCHC070103, NBCHC090055, 5-R01-AA012238, 5-R01-AA014886, and 1-R01-DA025074.
DISCLOSURE
J.M. Shoemaker has received research support from the US Department of Energy and the Suzie Poole Foundation. Dr. Holdsworth reports no disclosures. Dr. Aine serves on the editorial boards of Neuroinformatics, Open Neuroimaging Journal, and Open Medical Imaging Journal; has received research support from the NIH and UNM SOM, CTSC Pilot Project Grant Award. Dr. Calhoun has received research support from the NIH (NIMH, NIBIB, NCRR), National Science Foundation, and the US Department of Energy. R. de La Garza reports no disclosures. Dr. Feldstein Ewing has served as a consultant for DiClemente Research Group (UGA). Dr. Hayek reports no disclosures. Dr. Mayer serves as a consultant for Visionquest and has received research support from the NIH and the US Department of Energy. Dr. Kiehl has received research support from the NIH and the US Department of Energy. L.E. Petree reports no disclosures. Dr. Sanjuan receives research support from the NIH (NIAAA, NIDA) and the US Department of Energy. A. Scott reports no disclosures. Dr. Stephen receives research support from NIH. Dr. Phillips serves on the editorial board of the journal Pediatric Neurology; has received research support from the NIH and the Delle Foundation; and has given expert testimony in medico-legal cases.
REFERENCES
- 1. Wolf SM, Lawrenz FP, Nelson CA, et al. Managing incidental findings in human subjects research: analysis and recommendations. J Law Med Ethics 2008;36:219–248, 211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Orme NM, Fletcher JG, Siddiki HA, et al. Incidental findings in imaging research: evaluating incidence, benefit, and burden. Arch Intern Med 2010;170:1525–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morris Z, Whiteley WN, Longstreth WT, Jr, et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2009;339:b3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med 2007;357:1821–1828 [DOI] [PubMed] [Google Scholar]
- 5. Katzman GL, Dagher AP, Patronas NJ. Incidental findings on brain magnetic resonance imaging from 1000 asymptomatic volunteers. JAMA 1999;282:36–39 [DOI] [PubMed] [Google Scholar]
- 6. Illes J, Kirschen MP, Edwards E, et al. Ethics: incidental findings in brain imaging research. Science 2006;311:783–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Royal JM, Peterson BS. The risks and benefits of searching for incidental findings in MRI research scans. J Law Med Ethics 2008;36:305–314, 212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nelson CA. Incidental findings in magnetic resonance imaging (MRI) brain research. J Law Med Ethics 2008;36:315–319, 213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Illes J. ‘Pandora's box’ of incidental findings in brain imaging research. Nat Clin Pract Neurol 2006;2:60–61 [DOI] [PubMed] [Google Scholar]
- 10. Kirschen MP, Jaworska A, Illes J. Subjects' expectations in neuroimaging research. J Magn Reson Imaging 2006;23:205–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bockholt HJ, Scully M, Courtney W, et al. Mining the Mind Research Network: a novel framework for exploring large scale, heterogeneous translational neuroscience research data sources. Front Neuroinform 2010;3:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cramer SC, Wu J, Hanson JA, et al. A system for addressing incidental findings in neuroimaging research. Neuroimage 2011;55:1020–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Milstein AC. Research malpractice and the issue of incidental findings. J Law Med Ethics 2008;36:356–360, 214 [DOI] [PubMed] [Google Scholar]
- 14. Protection of human subjects; Belmont Report: notice of report for public comment. Fed Regist 1979;44:23191–23197 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.