Abstract
A survey of parasites of freshwater fishes in Kanjali and Harike wetlands of Punjab (India) revealed the presence of two new myxosporean species belonging to the genus Myxobolus Bütschli (Winter (ed): Protozoa, 1882). Spores of the first species, M. saugati sp. nov. parasitizing scales of Labeo rohita (Cypriniformis: Cyprinidae) vern. rohu are characterized in having ‘spores measuring 8.3 × 6.6 μm in size, oval to spherical in valvular view having rounded anterior and posterior ends; polar capsules are two, equal, measuring 4.0 × 2.4 μm, pyriform with distinct neck at the anterior end; an intercapsular process is absent’. Spores of the second species, Myxobolus szekeli sp. nov. parasitizing internal wall of stomach of Wallago attu (Cypriniformis: Siluridae) vern. mulle are ‘8.7 × 4.1 μm in size, elongately ovoidal in valvular view having tapering, bluntly pointed anterior end and rounded posterior end; polar capsules are two, equal, measuring 4.4 × 1.5 μm, tubular with blunt anterior and rounded posterior ends; polar capsules converge anteriorly but are placed at a distance posteriorly and occupies nearly half of the spore body cavity; an intercapsular process is absent’.
Keywords: Cypriniformes, Harike wetland, Kanjali wetland, L. rohita, W. attu
Introduction
Kanjali and Harike are major Wetlands spread over 185 and 4,100 ha area respectively in Punjab, India among other Wetlands (i.e. Ropar, Ranjit Sagar and Nangal wetlands) in the Ramsar list Wetlands of International importance. Harike wetland harbour 26 species and Kanjali wetland 17 species of freshwater fishes. These large varieties of fishes in these wetlands are vulnerable to various parasitic infections, out of which Myxozoa is emerging as the major group. Presently, there are more than 2,180 species in 62 genera belong to the Phylum Myxozoa has been described from fishes (Lom and Dykova 2006). Recently a new genus, Thelohanelloid bengalensis from gall bladder of Arius sagor (a marine fish in Bay of Bengal) has been described by Sarkar (2009). Out of this, 744 species belong to the genus Myxobolus and is the most common genus reported worldwide (Eiras et al. 2005). According to Kalavati and Nandi (2007) 104 species of Myxobolus spp. are restricted to the Indian sub-continent. Recently, Kaur and Singh (2008, 2009, 2010a, b, 2010/2011, 2011a, b, c, d, e) have contributed 15 new species to the genus Myxobolus from freshwater fishes in Wetlands of Punjab.
During the present investigation 62 fishes belonging to L. rohita and 25 fishes belonging to W. attu were examined to ascertain the prevalence of myxozoan parasites in these wetlands. A variety of other freshwater fishes were also collected and examined which include Cyprinus carpio, Catla catla, Amblypharyngodon mola, Labeo bata, Labeo dero and Mystus seenghala. Two new species M. saugati and M. szekeli are reported from scales and internal wall of stomach respectively. The description has been prepared according to the guidelines of Lom and Arthur (1989).
Materials and methods
Fishes collected from the Kanjali and Harike wetlands were brought to the laboratory and examined for myxozoan infections. Plasmodia when found were removed and teased on slide and covered with cover slip and examined under the oil immersion for the presence of myxospores. Fresh spores were treated with 8% KOH solution for the extrusion of polar filaments. For permanent preparation, air-dried smears were stained with Ziehl–Neelsen and iron–haematoxylin. Drawings were made from stained material with the aid of camera lucida. Measurement of spores was done with the aid of a calibrated ocular micrometer. All measurements are presented in μm as range values followed by mean ± SD in parentheses.
Results and discussion
M. saugati sp. nov.
Plasmodia
Large, white to pale yellow, present all over the scales, 10–12 in number and measure 1.0–1.5 mm in diameter. 15–18 Spores are present per plasmodium.
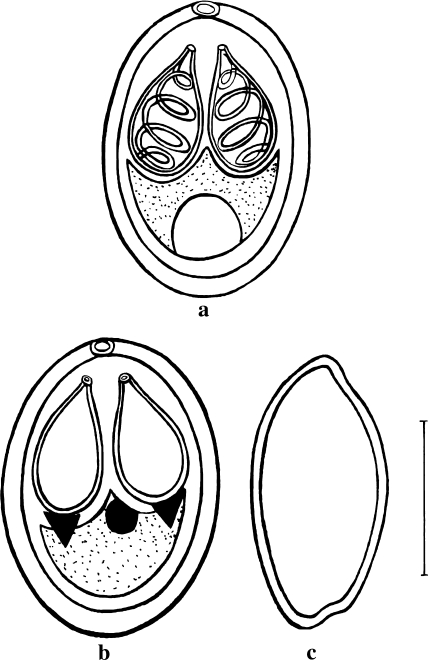
Spore description (Figs. 1a–c, 2a, b, 5a; Table 1) (measurements based on 8–9 spores in frontal view)
Fig. 1.
a Spore stained in Ziehl–Neelsen (valvular view). b Spore stained in iron–haematoxylin. c Spore in side view. Scale bar 0.005 mm
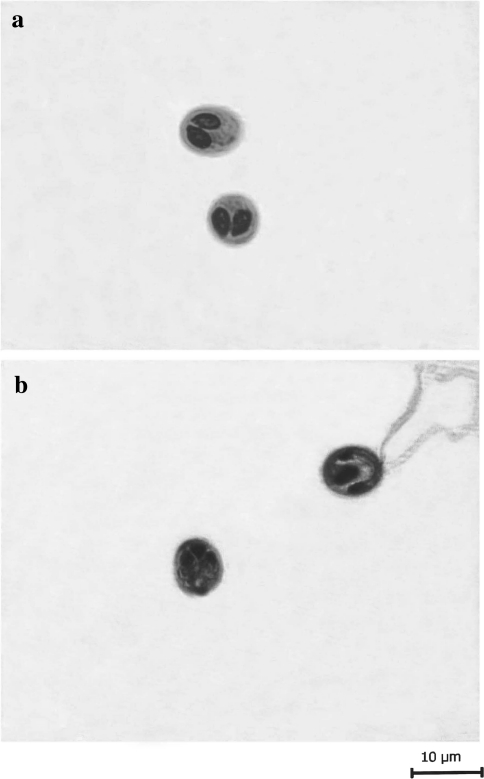
Fig. 2.
a Spores stained in Ziehl–Neelsen. b Spore stained in iron–haematoxylin (extructed polar filaments)
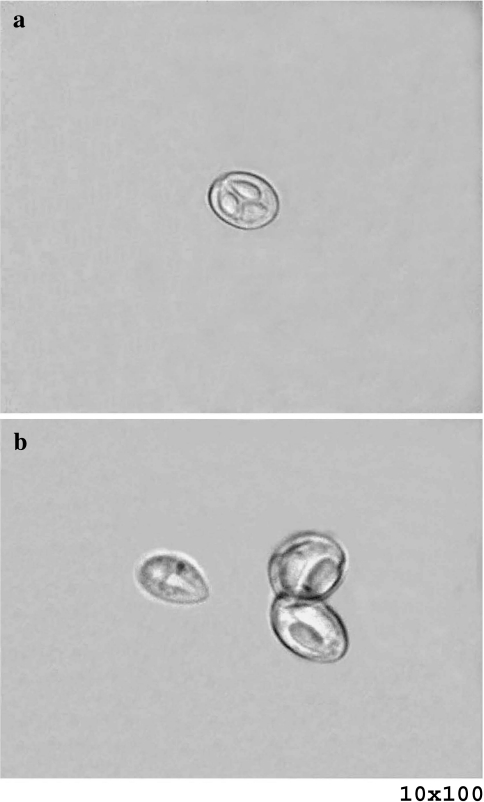
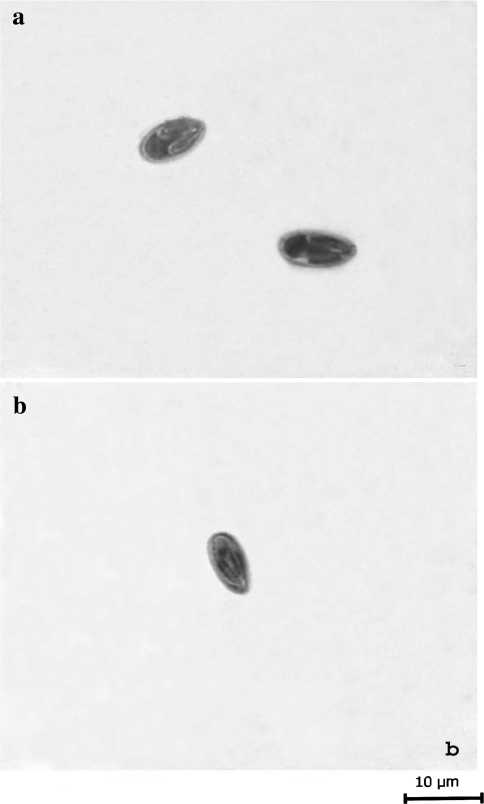
Fig. 5.
a Fresh spore of M. saugati sp. nov. b Fresh spores of M. szekeli sp. nov.
Table 1.
Measurements (in μm) and ratio of M. saugati sp. nov.
| Characters | Range | Mean values | SD |
|---|---|---|---|
| LS | 7.9–8.7 | 8.3 | 0.56 |
| WS | 6.4–6.8 | 6.6 | 0.28 |
| LPC | 3.7–4.3 | 4.0 | 0.42 |
| WPC | 2.0–2.8 | 2.4 | 0.56 |
| Ratio: LS/WS | 1.2 | ||
| ICP | Absent | ||
| NC | 4–5 | ||
| Parietal folds | Absent |
LS length of spore, WS width of spore, LPC length of polar capsule, WPC width of polar capsule, ICP intercapsular process, NC number of coils of polar filaments, SD standard deviation
The spores are histozoic, measure 8.3 × 6.6 μm, oval to spherical in valvular view having rounded anterior and posterior ends. Two shell valves are thick, smooth, symmetrical and measure 0.6 μm in thickness. Parietal folds are absent. Polar capsules are two, equal, measure 4.0 × 2.4 μm and are pyriform with distinct neck at the anterior end. They converge anteriorly and are placed at a distance posteriorly. Polar filaments form 4–5 coils and are arranged obliquely to the polar capsule axis. They are equal when extruded and measure 26.6 μm in length. An intercapsular process is absent. Two capsulogenic nuclei are present beneath each polar capsule measuring 1.50 μm in diameter. Sporoplasm agranular, homogenous occupying whole of the extracapsular space behind the polar capsules and contain one nucleus and a large iodinophilous vacuole measuring 1.6 and 2.5 μm in diameter respectively.
Taxonomic summary of M. saugati sp. nov.
| Type host | Labeo rohita vern. rohu |
| Type locality | Kanjali wetland, Punjab, India |
| Type specimen | Paratypes are spores stained in Ziehl–Neelsen and iron–haematoxylin, deposited in the museum of department of Zoology, Punjabi University, Patiala. Slide no. CM/P/ZN/21.04.2009 and CM/P/IH 11.08.2009. |
| Site of infection | Scales |
| Prevalence of infection | 56.4% (35/62) |
| Etymology | The species epithet saugati has been given after the name of the Dr. Saugata Basu, an eminent worker of Protozoology Laboratory, Department of Zoology, University of Kalyani, Kalyani, West Bengal, India. |
Discussion
The present species under study was compared with M. pfeifferi, M. minutus, M. catostomi, M. catlae, M. sangoricus, M. baueri, M. lotae, M. strelkovi, M. bhadrensis, M. shetii, M. rohitae, M. gobiorum, M. dermatis, M. haldari, M. cartilaginis, M. serrata, M. kwangsiensis, M. nkolyaensis, M. edellae, M. shantipuri, M. chilkensis, M. tingrelaensis, M. sushmii, M. punjabii and M. patialensis but differs from all of the above species in morphometrics (Table 2).
Table 2.
Comparative description of M. saugati sp. nov. with morphologically similar species (measurements are in micrometer)
| Species | Host | Site of infection | Locality | Spore | Polar capsule |
|---|---|---|---|---|---|
| M. saugati sp. nov. (present study) | Labeo rohita | Scales | Kanjali wetland Punjab (India) | 8.3 × 6.6 | 4.0 × 2.4 |
| M. pfeifferi Thelohan 1895 | Barbus barbus | Kidney, spleen, muscles, gills | – | 10.0–13.0 × 9.0–12.2 | 5.0–5.7 |
| M. minutus Nemeczek 1911 | Leuscus leuscus, L. cephalus | Gills | Germany | 6.0 × 4.2–5.0 | 3.0 × 2.0 |
| M. catostomi Kudo 1923 | Catastomus commersonii | Muscles | Canada | 10.0–12.7 × 7.6–10.0 | 3.6–5.5 × 1.3–2.7 |
| M. catlae Chakravarty 1943 | L. rohita | Gills | West Bengal (India) | 14.5–16.5 × 6.18 | 10.3–12.3 × 2.06–3.01 |
| M. sangoricus Gogebashvili 1966 | Varicorhynus capoeta | Fin, gills, kidney | Caucasus | 9.1–11.0 × 7.8–9.2 | 3.1–3.9 × 1.8–3.0 |
| M. baueri Chernova 1970 | Tinca tinca | Gills, heart, kidney, liver | Russia | 14.0–16.0 × 9.3–12.0 | 5.3–6.0 × 2.7–3.3 |
| M. lotae Mitenev 1971 | Lota lota | Gills | Russia | 8.4–10.4 × 6.2–6.5 | 3.4–4.4 × 2.3–3.4 |
| M. strelkovi Kostarev and Kulemina 1971 | Phoxinus phoxinus, Leuciscus idus | Gills, liver | Russia | 8.0–12.2 × 6.0–11.0 | 3.3 × 5.4 × 2.0–4.1 |
| M. bhadrensis Seenappa and Manohar 1981 | L. rohita | Muscle | Karnataka (India) | 9.5 × 7.14 | 3.5 × 2.2 and 2.5 × 1.75 |
| M. shetti Seenappa and Manohar 1981 | Cirhina mrigala | Gills | Karnataka (India) | 8.8 × 7.4 | 3.4 × 2.3 |
| M. rohitae Haldar et al. 1983 | L. rohita, L. bata, L. dyocheilus, L. calbasu, Puntius sarana | Scales | West Bengal (India) | 10.6 × 9.0 | 6.6 × 3.3 |
| M. gobiorum Donec 1984 in Shulman | Gobio gobio | Fin | Ukraine | 11.0–13.0 × 9.0–10.0 | 5.2–6.5 × 2.2–3.0 |
| M. dermatis (Haldar et al. 1981) Gupta and Khera 1988 | L. rohita | Scales | West Bengal Kerala (India) | 10.3 × 9.4 | 4.4 × 2.2 |
| M. haldari Gupta and Khera 1989 | L. rohita | Fin, gills | Punjab (India) | 9.31 × 7.95 | 4.31 × 2.97 and 2.95 × 1.98 |
| M. cartilaginis (Hoffman et al. 1965) Landsberg and Lom 1991 | Lepomis macrochirus | Head cartilage | USA | 10.2 × 8.9 | 5.3 × 3.3 |
| M. serrata Pagarkar and Das 1993 | Cyprinus carpio | Gill arch | West Bengal (India) | 9.07 × 8.5 | 4.47 × 2.6 |
| M. kwangsiensis Hsieh et al. 1993 | L. rohita | Gills, kidney | China | 10.9 × 6.9 | 5.5 × 2.9 |
| M. nkolyaensis Fomena and Bouix 1994 | Barbus jae | Caudal muscles | Cameroon | 9.0 × 8.3 | 4.4 × 3.0 |
| M. edellae Sarkar 1999 | Ctenopharyngodon idella | Kidney | West Bengal (India) | 10.7 × 9.4 | 6.14 × 3.12 |
| M. shantipuri Basu and Haldar 2002 | Catla catla × L. rohita | Gill lamellae | West Bengal (India) | 7.3 × 5.8 | 4.0 × 2.4 |
| M. tingrelaensis Boungou et al. 2006 | Sarotherodon galilaeus | Among the rays of fin | West Africa | 11.6 × 9.37 | 4.80 × 2.40 |
| M. chilkensis (Kalavati et al. 1992) Kalavati and Nandi 2007 | L. rohita | Gall bladder | Orissa (India) | 7.2–8.0 (7.77) × 5.6–6.6 (6.2) | 3.2–4.8 (4.2) × 1.8–2.2 (1.98) and 1.0–1.2 (1.08) |
| M. sushmii Kaur and Singh 2010/2011 | L. rohita | Eye ball | Harike wetland, Punjab (India) | 9.0–10.2 (9.6) × 7.3–9.3 (8.3) | 4.1–5.3 (4.70) × 2.9–3.5 (3.20) and 2.9–3.4 (3.10) × 1.0–3.0 (2.0) |
| M. punjabii Kaur and Singh 2010/2011 | L. rohita | Caudal fin | Kanjali Wetland Punjab (India) | 8.1–9.7 (8.9) × 6.4–7.5 (6.9) | 3.4–5.4 (4.4) × 2–3.4 (2.7) and 2.8–3.6 (3.2) × 1.2–2.2 (1.7) |
| M. patialensis Kaur and Singh 2011e | L. rohita | Caudal fin | Ropar wetland, Punjab (India) | 11.28 × 6.67 | 4.8 × 3.1 and 1.70 × 1.51 |
The present species is oval to spherical in shape with rounded anterior and posterior extremities without any parietal folds. They contain equal, pyriform polar capsules with anterior ends terminating into a distinct neck. In this respect, it is comparable with the spores of M. minutus, M. catostomi, M. sangoricus, M. baueri, M. lotae, M. tingrelaensis, M. shantipuri, M. rohitae, M. shetii, M. strelkovi and M. pfeiferri. An intercapsular process is absent in the species under study. But the presence of intercapsular process in M. strelkovi, M. pfeiferri, M. tingrelaensis, M. shetii, M. sangoricus, M. baueri, M. catostomi, M. minutus, M. lotae and a triangular notch in M. rohitae differentiates all of the above species from the present species. Ovoidal or tear-shaped spores of M. shantipuri with either equal or unequal polar capsules can also be differentiated from the spores of the present species.
In view of the above differences, the present species under study is proposed as new to the science and named as M. saugati sp. nov. through this communication.
M. szekeli sp. nov
Plasmodia
White, spherical to rounded, present on the internal wall of the stomach, 1–2 in number and measure 0.6–0.8 mm in diameter. 5–6 spores are present per plasmodium.
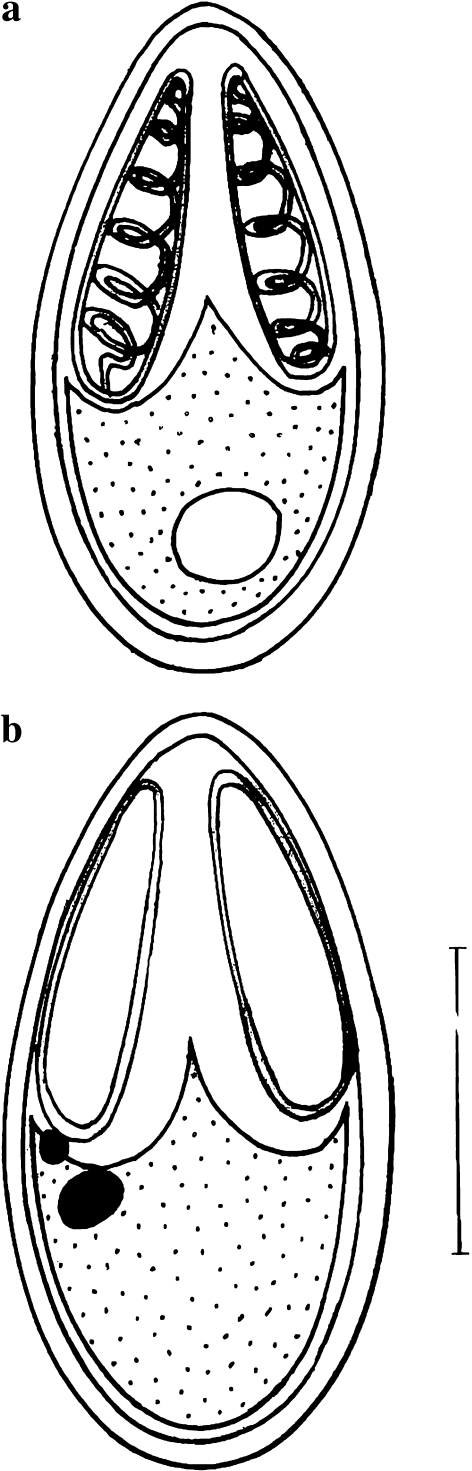
Spore description (Figs. 3a, b, 4a, b, 5b; Table 3) (measurements based on 4–8 spores in frontal view)
Fig. 3.
a Spore stained in Ziehl–Neelsen (valvular view). b Spore stained in iron–haematoxylin. Scale bar 0.005 mm
Fig. 4.
a Spore stained in Ziehl–Neelsen. b Spore stained in iron–haematoxylin
Table 3.
Measurements (in μm) and ratio of M. szekeli sp. nov.
| Characters | Range | Mean values | SD |
|---|---|---|---|
| LS | 8.4–9.0 | 8.7 | 0.42 |
| WS | 3.9–4.3 | 4.1 | 0.28 |
| LPC | 3.9–4.9 | 4.4 | 0.70 |
| WPC | 1.0–2.0 | 1.5 | 0.70 |
| Ratio: LS/WS | 2.1 | ||
| ICP | Absent | ||
| NC | 5–6 | ||
| Parietal folds | Absent |
The spores are histozoic, measure 8.7 × 4.1 μm, elongately ovoidal in valvular view having tapering, bluntly pointed anterior end and rounded posterior end. Two shell valves are thin, smooth, symmetrical and measure 0.3 μm in thickness. Parietal folds are absent. Polar capsules are two, equal, measure 4.4 × 1.5 μm, tubular with blunt anterior and rounded posterior ends. Polar capsules converge anteriorly but are placed at a distance posteriorly and occupies nearly half of the spore body cavity. Polar filaments form 5–6 coils and are arranged perpendicular to the polar capsule axis. An intercapsular process is absent. One capsulogenic nucleus is present measuring 1.3 μm in diameter. Sporoplasm is agranular and homogenous occupying whole of the extracapsular space behind the polar capsules. One sporoplasmic nucleus measuring 1.4 μm in diameter is present. An iodinophilous vacuole is present.
Taxonomic summary of M. szekeli sp. nov.
| Type host | Wallago attu vern. mulle |
| Type locality | Harike wetland, Punjab, India |
| Type specimen | Paratypes are spore stained in Ziehl–Neelsen and iron–haematoxylin, deposited in the museum of department of Zoology, Punjabi University, Patiala. Slide no. MH/ZN/01.03.2010 and MH/IH/01.03.2010. |
| Site of infection | Internal wall of the stomach |
| Prevalence of infection | 4% (1/25) |
| Etymology | The species epithet szekeli has been given after the name of Dr. Csaba Szekely of Veterinary Medical Research Institute, Hungarian Academy of Sciences, Hungary. |
Discussion
The present species was compared with M. aligarhensis, M. mathuri, M. hosadurgensis, M. amieti, M. attui, M. bhadurius, M. trichogasteri, M. scatophagi, M. variformis, M. orissae and M. dhanachandi but differ from all of the above species in morphometric characteristics (Table 4).
Table 4.
Comparative description of M. szekeli sp. nov. with morphologically similar species (measurements in micrometer)
| Species | Host | Site of infection | Locality | Spore | Polar capsule |
|---|---|---|---|---|---|
| M. szekeli sp. nov. (present study) | Wallago attu | Internal wall of the stomach | Harike wetland, Punjab (India) | 8.7 × 4.1 | 4.4 × 1.5 |
| M. aligarhensis Bhatt and Siddiqui 1964 | Channa punctatus | Accessory respiratory membrane, pharyngeal epithelium, fin | West Bengal (India) | 12.0–14.0 × 6.0–7.5 | 6.5–8.0 × 2.0–2.5 and 6.0–7.0 × 2.5 |
| M. hosadurgensis Seenappa and Manohar 1981 | Cirrhina mrigala | Gill, muscles | Karnataka (India) | 10.5 × 6.25 | 5.37 × 2.3 and 3.3 × 1.43 |
| M. mathuri Jayasri et al. 1981 | Puntius sarana | Gills | Rajasthan (India) | 8.7–23.5 × 5.1–10.1 | 2.7–11.9 × 1.8–4.6 and 2.7–7.8 × 1.8–4.6 |
| M. amieti Fomena et al. 1985 | Ctenopoma nanum | Spleen, eye | Cameroon | 14.0 × 7.4 | 8.4 × 1.9 |
| M. attui Sarkar 1985a | W. attu | Gut | West Bengal (India) | 13.89 × 8.53 | 5.92 × 3.0 |
| M. bhadurius (Sarkar 1985a) Gupta and Khera 1988 | W. attu | Gall bladder | West Bengal (India) | 10.59 × 6.28 | 5.31 × 2.78 |
| M. trichogasteri (Sarkar 1985b) Gupta and Khera 1988 | Trichogaster fasciatus | Gall bladder | West Bengal (India) | 15.55 × 9.35 | 10.1 × 3.32 |
| M. scatophagi Haldar et al. 1996 | Scatophagus argus | Gills | Orissa (India) | 14.0 × 5.3 | 7.13 × 2.36 |
| M. variformis Haldar et al. 1996 | Mystus gulio | Body muscles, gills | Orissa (India) | 15.2 × 5.65 | 9.67 × 2.84 and 8.6 × 2.28 |
| M. orissae Haldar et al. 1996 | C. mrigala | Gills | Orissa (India) | 15.71 × 6.8 | 8.8 × 1.78 and 7.58 × 2.57 |
| M. dhanachandi Bandyopadhyay et al. 2007 | Channa orientalis | Dorsal, ventral and caudal fins | Manipur (India) | 18.4 × 6.05 | 8.26 × 1.43 |
The shape of the present species is elongately ovoidal with tapering, blunt anterior end and rounded posterior end. In this respect, the present species can be compared with M. aligarhensis, M. hosadurgensis and M. amieti in having spores with blunt anterior end as spores in rest of other species are pointed anteriorly. The species under study was also closely compared with M. dhanachandi on the basis of morphological similarities. But spores in M. dhanachandi are dumb-bell shaped, broader at the middle and tapering at both ends. Polar capsules are two, equal, tubular with blunt anterior but rounded posterior end and occupies nearly half of the spore body cavity in species under study. Unequal polar capsule in M. aligarhensis and M. hosadurgensis, very elongate polar capsules reaching two-third of the spore length in M. amieti and tear shaped polar capsules with anteriorly sharply pointed ends in M. dhanachandi differentiate them from the present species under consideration. In addition, both polar capsules in the present species converge anteriorly but are placed at a distance posteriorly in contrast to almost parallel polar capsules in M. dhanachandi.
In view of the above differences, the present species under study is proposed as new to the science and named as M. szekeli sp. nov. through this communication.
References
- Bandyopadhyay PK, Hemananda T, Mitra AK, Mohilal N. Myxobolus dhanachandi sp. n. (Myxozoa, Myxosporea, Bivalvulida) from an Indian freshwater fish Channa orientalis (Bloch-Schneider) Protistology. 2007;14(4):353–356. [Google Scholar]
- Basu S, Haldar DP. Observations on three new species of Myxobolus Butschli, 1882 from hybrid carps of West Bengal, India. Indian J Environ Ecoplan. 2002;6(3):629–640. [Google Scholar]
- Bhatt VS, Siddiqui WA. Four new species of Myxosporidia from the Indian fresh water fish Ophiocephalus punctatus Bloch. J Protozool. 1964;11:314–316. [Google Scholar]
- Boungou M, Kabre GB, Sakiti NG, Marque A, Sawadogo L. Description of four new Myxosporeans (Myxozoa: Myxosporean) from genus Myxobolus, fish parasites of Burkina Faso, West Africa. J Biol Sci. 2006;6(5):861–867. doi: 10.3923/jbs.2006.861.867. [DOI] [Google Scholar]
- Bütschli O (1882) Myxosporidia. In: Winter CF (ed) Bronn’s Klassens und Ordnungen des Tierreichs, vol I Protozoa, 2nd edn. Leipzig, pp 590–603
- Chakravarty M. Studies on myxosporidia from the common food fishes of Bengal. Proc Indian Acad Sci. 1943;18:21–35. [Google Scholar]
- Chernova TN. New species of mucous sporozoans (Myxosporidia) of fish in some reservoirs of western Georgia. Vestnik Zool. 1970;2:60–64. [Google Scholar]
- Donec ZS (1984) In: Shulman SS (ed) Parasitic protozoa. In: Bauer ON (ed) Key to parasites of freshwater fish of the USSR, Leningrad: ‘‘Nauka’’, vol 1, pp 426
- Eiras JC, Molnar K, Lu YS. Synopsis of the species of Myxobolus Butschli, 1882 (Myxozoa: Myxosporea: Myxobolidae) Syst Parasitol. 2005;61:1–46. doi: 10.1007/s11230-004-6343-9. [DOI] [PubMed] [Google Scholar]
- Fomena A, Bouix G. New myxosporidia species from freshwater water teleosts in Southern Cameroon (Central Africa) J Afr Zool. 1994;108:481–491. [Google Scholar]
- Fomena A, Bouix G, Birgi E. Contribution a l’etude des Myxosporidiies des poissons d’eau douc du Cameroun. II. Especes nouvelles de genres Myxobolus Butschli, 1882. Bull Inst Fond Afr Noire. 1985;46:167–192. [Google Scholar]
- Gogebashvili IV. Parasitic protozoans of fish in the Kura River (within the boundaries of Georgia) Bull Acad Sci Ga. 1966;43:465–472. [Google Scholar]
- Gupta S, Khera S (1988) Review of the genus Myxobolus Butschli, 1882. Res Bull (Sci) Panj Univ 39(I–II):45–48
- Gupta S, Khera S. Observations on Myxobolus haldari sp. nov. (Myxozoa: Myxosporea) from freshwater fishes of North India. Res Bull (Sci) Panj Univ. 1989;40:281–291. [Google Scholar]
- Haldar DP, Mukherjee M, Kundu TK. Observations on two new species of Myxosoma Thelohan, 1892 (Myxozoa: Myxosomatidae) from fresh water teleost fishes. Arch Protistenkd. 1981;124:244–251. doi: 10.1016/S0003-9365(81)80016-4. [DOI] [Google Scholar]
- Haldar DP, Das MK, Sharma BK. Studies on protozoan parasites from fishes. Four new species of the genera Henneguya Thelohan, 1892, Thelohanellus Kudo, 1933 and Myxobolus Butschli, 1892. Arch Protistenkd. 1983;127:283–296. doi: 10.1016/S0003-9365(83)80023-2. [DOI] [Google Scholar]
- Haldar DP, Samal KK, Mukhopadhyaya D. Studies on protozoan parasites of fishes in Orissa: eight species of Myxobolus Butschli (Myxozoa: Bivalvulida) J Bengal Nat Hist Soc. 1996;16:3–24. [Google Scholar]
- Hoffman GL, Putz RE, Dunbar CE. Studies on Myxosoma cartilaginis n. sp. (Protozoa: Myxosporidia) of centrarchid fish and a synopsis of the Myxosoma of North American freshwater fishes. J Protozool. 1965;12:319–332. [Google Scholar]
- Hsieh XR, Gong XN, Xiao WH. Descriptions of a new model of myxosporean sporogenesis and a new species. Trans Res Fish Dis. 1993;1:77–82. [Google Scholar]
- Jayasri M, Parvateesam M, Mathur PN. Myxosoma mathurii n. sp. (Protozoa: Myxosporidia) parasitic on Puntius sarana (Ham.) Curr Sci. 1981;50:16. [Google Scholar]
- Kalavati C, Nandi NC. Handbook of Myxosporidean parasites of Indian fishes. India, Kolkata: ZSI; 2007. [Google Scholar]
- Kalavati C, Venkateswara Rao J, Vaidaehi J. Myxosporidian parasites (Protozoa) of fishes of Chilka lake, east coast of India: three new species of Henneguya, Thelohan, Rudicapsula, Kalavati and Narasimhamurti and Unicauda, Auerbachi. Indian J Parasitol. 1992;16(1):77–83. [Google Scholar]
- Kaur H, Singh R (2008) Observations on one new species, of Genus Myxobolus—M. naini and redescription of M. magauddi recorded from freshwater fishes of Kanjali Wetland of Punjab, India. Proc 20th natl congr Parasitol, pp 75–79
- Kaur H, Singh R. A new myxosporean species, Myxobolus eirasi sp nov, a known species M. venkateshi Seenappa, Manohar (1981) from the Indian major carp fish Cirrhina mrigala (Ham) Protistology. 2009;6(2):126–130. [Google Scholar]
- Kaur H, Singh R. A new myxosporean species Myxobolus sclerii sp. nov. and one known species M. stomum Ali et al. (2003) from two Indian major carp fishes. J Parasit Dis. 2010;34(1):33–39. doi: 10.1007/s12639-010-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Singh R. One new myxosporidian species, Myxobolus slendrii sp. nov., one known species, M. punjabensis Gupta, Khera (1989) infecting freshwater fishes in wetlands of Punjab, India. Parasitol Res. 2010;106(5):1043–1047. doi: 10.1007/s00436-010-1746-9. [DOI] [PubMed] [Google Scholar]
- Kaur H, Singh R (2010/2011) Two new species of Myxobolus (Myxosporea, Bivalvulida) from the Indian major carp Labeo rohita Hamilton, 1822. Protistology 6(4):264–270
- Kaur H, Singh R (2011a) Two new species of Myxobolus (Myxozoa: Myxosporea: Bivalvulida) from freshwater fishes of Punjab Wetlands (India). J Parasit Dis. doi:10.1007/s12639-011-0024-9 [DOI] [PMC free article] [PubMed]
- Kaur H, Singh R (2011b) Two new species of Myxobolus (Myxozoa: Myxosporea: Bivalvulida) infecting an Indian major carp in Ropar and Kanjali wetlands (Punjab). J Parasit Dis. doi:10.1007/s12639-011-0033-8 [DOI] [PMC free article] [PubMed]
- Kaur H, Singh R (2011c) Myxobolus harikensis sp. nov. (Myxozoa: Myxobolidae) infecting fins of Cirrhina mrigala (Ham.)—an Indian major carp in Harike Wetland, Punjab (India). Parasitol Res. doi:10.1007/s00436-011-2445-x [DOI] [PubMed]
- Kaur H, Singh R (2011d) Two new and one already known species of Myxobolus (Myxozoa: Myxosporea: Bivalvulida) infecting gill lamellae of Indian major carp fishes in Ropar and Harike wetlands (Punjab). Proceedings of the 22nd National Congress on Parasitology (accepted)
- Kaur H, Singh R (2011e) Two new species of Myxobolus (Myxozoa: Myxosporea: Bivalvulida) infecting Indian freshwater fishes in Punjab Wetlands (India). Parasitol Res. doi:10.1007/s00436-011-2307-6 [DOI] [PubMed]
- Kostarev GF, Kulemina IV (1971) A new species of Myxobolus in fish. Vestnik Leningradskogo Universita, Seriya Biologii 9:145–146
- Kudo R. Development of a myxosporidian, Myxosoma catostomi nov. spec. Anat Rec. 1923;24:39. [Google Scholar]
- Landsberg JH, Lom J. Taxonomy of the genera of the Myxobolus/Myxosoma group (Myxobolidae: Myxosporea), current listing of species and revision of synonyms. Syst Parasitol. 1991;18:155–186. doi: 10.1007/BF00009358. [DOI] [Google Scholar]
- Lom J, Arthur JR. A guideline for the preparation of species descriptions in Myxosporea. J Fish Dis. 1989;12:151–156. doi: 10.1111/j.1365-2761.1989.tb00287.x. [DOI] [Google Scholar]
- Lom J, Dykova I. Myxozoan genera: definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol. 2006;53:1–36. [PubMed] [Google Scholar]
- Mitenev VK. New and little known species of myxosporidians from fishes of the Kola Peninsula. Parazitologiya. 1971;5:556–558. [Google Scholar]
- Nemeczek A. Beitra ge zur Kenntnis der Myxo-und Microsporidien der Fische. Arch Protistenkd. 1911;22:143–169. [Google Scholar]
- Pagarkar AU, Das M. Two new species of myxozoa, Thelohanellus caudatus n. sp. and Myxobolus serrata n. sp. from cultural carps. J Inland Fish Soc India. 1993;25(1):30–35. [Google Scholar]
- Sarkar NK. Some coelozoic Myxosporida (Myxozoa: Myxosporea) from a freshwater water teleost fish of River Padma. Acta Protozool. 1985;24(1):47–53. [Google Scholar]
- Sarkar NK. Some Myxosporidia (Myxozoa: Myxosporea) of Anabantid fishes of West Bengal, India. Acta Protozool. 1985;24(2):175–180. [Google Scholar]
- Sarkar NK. Some new myxosporidia (Myxozoa: Myxosporea) of the genera Myxobolus Butschli 1882, Unicapsula Davis 1942, Kudoa Meglitsch 1947, Ortholinea, Shulman 1962 and Neaparvicapsula Gaevskaya, Kovaleva and Shulman, 1982. Proc Zool Soc Calcutta. 1999;52(1):38–48. [Google Scholar]
- Sarkar NK. Thelohanelloid bengalensis gen. and sp. nov. (Myxosporea: Thelohanellidae) from the gall bladder of marine catfish of the Bay of Bengal, India. Uttar Pradesh J Zool. 2009;29(2):251–254. [Google Scholar]
- Seenappa D, Manohar L. Five new species of Myxobolus (Myxosporea: Protozoa), parasitic in Cirrhina mrigala (Hamilton) and Labeo rohita (Hamilton), with a note on a new host record for M. curmucae Seenappa and Manohar, 1980. J Protozool. 1981;28:358–360. [Google Scholar]
- Thelohan P. Recherches sur les Myxosporidies. Bull Sci Fr Belg. 1895;26:100–394. [Google Scholar]