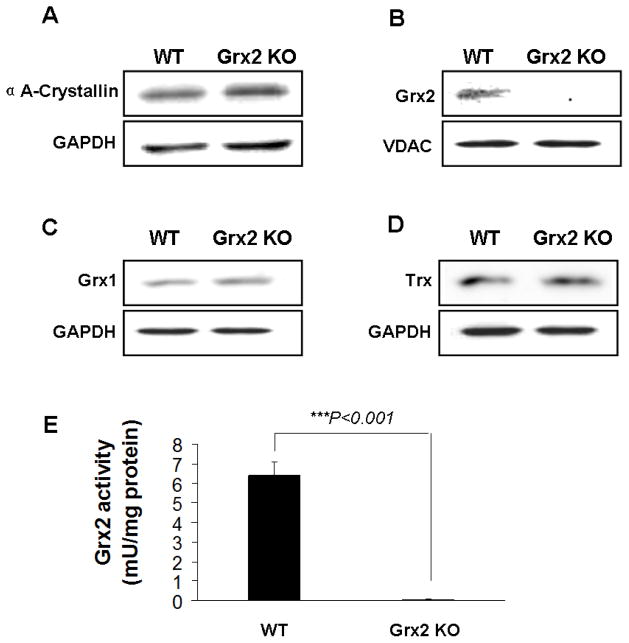
Fig. 1. Characterization of wild type (WT) and Grx2 knockout Grx2 KO) primary mouse lens epithelial cells (LEC)s.
Primary mouse LECs were isolated from WT and Grx2 KO lens epithelial layers. (A) Expression of αA-crystallin in wild type and Grx2 knockout LECs. Whole cell lysates (40 μg) were obtained from wild type or Grx2 knockout mouse LECs and analyzed by immunoblot by using anti-αA- crystallin antibody. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. (B) Detection of Grx2 in WT and Grx2 KO mouse LECs. 40 μg of mitochondrial isolated from WT and Grx2 KO cells were analyzed by Western blot with anti-Grx2 antibody. Voltage-dependent anion channels (VDAC) was used as both a mitochondrial marker and loading control. (C) Expression of glutaredoxin 1 (Grx1) in WT and Grx2 KO LECs. 40 μg of whole cell lysates obtained from WT or Grx2 KO cells were analyzed by immunoblot by using anti-glutaredoxin antibody. GAPDH was used as a loading control. (D) Expression of thioredoxin (Trx) in WT or Grx2 KO LECs. 40 μg of whole cell lysates obtained from wild type or Grx2 knockout cells were analyzed by immunoblot by using anti-thioredoxin antibody. GAPDH was used as a loading control. (E) Grx2 activity in wild type and Grx2 knockout LECs. Mitochondrial fraction isolated from WT or Grx2 KO cells were used to detect Grx2 activity as described under “Materials and methods”. Error bars indicate S.D., n=3, *P<0.05 vs. WT.