Abstract
AIM: To analyze the outcome of hepatocellular carcinoma (HCC) resection in cirrhosis patients, related to presence of portal hypertension (PH) and extent of hepatectomy.
METHODS: A retrospective analysis of 135 patients with HCC on a background of cirrhosis was submitted to curative liver resection.
RESULTS: PH was present in 44 (32.5%) patients. Overall mortality and morbidity were 2.2% and 33.7%, respectively. Median survival time in patients with or without PH was 31.6 and 65.1 mo, respectively (P = 0.047); in the subgroup with Child-Pugh class A cirrhosis, median survival was 65.1 mo and 60.5 mo, respectively (P = 0.257). Survival for patients submitted to limited liver resection was not significantly different in presence or absence of PH. Conversely, median survival for patients after resection of 2 or more segments with or without PH was 64.4 mo and 163.9 mo, respectively (P = 0.035).
CONCLUSION: PH is not an absolute contraindication to liver resection in Child-Pugh class A cirrhotic patients, but resection of 2 or more segments should not be recommended in patients with PH.
Keywords: Liver surgery, Hepatic resection, Hepatocellular carcinoma, Portal hypertension
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer death worldwide[1-3]. Surgical treatment is an effective treatment for HCC and the mortality after surgery has decreased in recent years in relation to improved surgical techniques and peri-operative management of patients[4-6]. The overall survival at 5 years after liver resection varies from 33% to 69% according to recent surgical series, although recurrence is still the major issue after surgery[7-13].
The indications for surgical resection depend on the characteristics of the tumor (stage, number of nodules, size, presence of vascular invasion), on the general condition of patient, and on liver functional reserve[14].
The presence of liver cirrhosis is the most important risk factor for the development of HCC, as 85%-95% of HCCs arise in cirrhotic livers[15-17]. HBV and HCV infections and alcohol abuse are the most frequent causes of cirrhosis: about 80%-85% of cases[7]. Portal hypertension (PH) is related to an increase in intrahepatic resistance due to the structural subversion of the liver and loss of vascular bed, and bleeding from gastroesophageal varices is one of the most important complications of cirrhosis[18]. On the basis of several studies[19,20], the American Association for the Study of Liver Diseases (AASLD)[21] defined as clinical PH the presence of esophageal varices or thrombocytopenia (platelet count < 100 000/mm3) associated with splenomegaly. The European Association for the Study of the Liver (EASL)/AASLD guidelines consider PH as a relative contraindication to liver resection, because of the high risk of postoperative liver failure, as reported in some clinical series[21,22]. These results, however, have not been confirmed in more recent clinical studies[23-25].
The aims of this study are to assess the results of liver resection in patients with HCC and cirrhosis with PH and the relationship in terms of survival between Child-Pugh stage, the extent of hepatic resection and the presence of clinical PH.
MATERIALS AND METHODS
We retrospectively analyzed clinical data of 135 patients with cirrhosis undergoing liver resection with radical intent for HCC from 1995 to 2008 at the single Surgical Division of the Department of Surgery of the University of Verona. The patients’ liver function was assessed by Child-Pugh classification. Clinical PH was defined according to AASLD guidelines as the presence of esophageal varices or thrombocytopenia (platelet count < 100 000/mm3) associated with splenomegaly[21]. The extent of resection was defined according to the classification of Brisbane[26]. After liver resection, patients underwent follow-up with serum α-fetoprotein levels and abdominal ultrasound every 6 mo and computed tomography scan every 12 mo. The mean follow-up after surgery was 38.3 mo.
Statistical analysis
Data were collected and analyzed with SPSS statistical software (SPSS version 16.0 Inc., Chicago III). The differences between categorical variables were analyzed with a χ2 test. The differences between continuous variables were also analyzed with a χ2 test.
Survival analysis was carried out with the Kaplan-Meier method. Univariate analysis for survival was performed with the Kaplan Meier method, with the Log Rank test to verify significance of differences. The statistical analysis included two different steps; in the first we analyzed the prognostic significance of the PH in all patients and in the second step we analyzed the prognostic significance of PH in different subgroups according to the Child-Pugh class and the extent of liver resection (wedge/segmentectomy or ≥ 2 segments). Finally, multivariate analysis with Cox’s regression model was performed with the following variables: Child-Pugh class, PH and type of hepatectomy. A P value lower than 0.05 was considered significant.
RESULTS
The prevalence of PH in all patients was 32.5%. The analysis of our data showed that patients with PH who underwent surgery had worse liver function compared to those without PH (patients in Child-Pugh B class 33% vs 11% respectively, P < 0.01), with serum bilirubin level > 2 mg/dL in 29% vs 3% respectively, P < 0.01 and serum transaminases AST > 80 U/L in 52% vs 25%, P = 0.01 and ALT > 80 U/L in 48% vs 19% respectively, P = 0.01 (Table 1). The one and 3-mo mortality rates were 4.6% and 13.9% and 1.1% and 3.3% for patients with and without PH, respectively (P = 0.20 and P = 0.05). The morbidity rate reached no statistical significance for patients with and without PH respectively (37% vs 32%, P = 0.59). The liver-related morbidity (ascites, encephalopathy, jaundice) was significantly higher in patients with PH than in patients without PH, 32% vs 13% respectively (P = 0.03) (Table 2).
Table 1.
Patients characteristics according to the presence/absence of portal hypertension
| Variable |
Portal hypertension |
Pvalue | |
| Yes (%) | No (%) | ||
| n | 44 | 91 | |
| Age (yr) | 0.55 | ||
| < 70 | 31 (70) | 60 (66) | |
| > 70 | 13 (30) | 31 (34) | |
| Etiology of liver disease | 0.21 | ||
| Alcohol | 7 (16) | 24 (26) | |
| Viral hepatitis | 36 (82) | 59 (65) | |
| Other | 1 (2) | 8 (9) | |
| Serum ALT level (U/L) | 0.01 | ||
| < 80 | 23 (52) | 74 (81) | |
| > 80 | 21 (48) | 17 (19) | |
| Serum AST level (U/L) | 0.01 | ||
| < 80 | 21 (48) | 68 (75) | |
| > 80 | 23 (52) | 23 (25) | |
| Child-Pugh class | 0.01 | ||
| Class A | 29 (66) | 81 (89) | |
| Class B | 15 (33) | 10 (11) | |
| Bilirubin level (mg/dL) | 0.01 | ||
| < 2 | 31 (71) | 88 (97) | |
| 2-3 | 8 (18) | 3 (3) | |
| > 3 | 5 (11) | 0 (0) | |
| Albumin level (g/L) | 0.01 | ||
| < 28 | 7 (16) | 8 (9) | |
| 28-35 | 18 (41) | 14 (15) | |
| > 35 | 19 (43) | 69 (76) | |
| Platelet count | 0.01 | ||
| ≤ 100 000/mm3 | 31 (70) | 0 | |
| > 100 000/mm3 | 13 (30) | 91 (100) | |
| Esophageal varices | 0.01 | ||
| Yes | 17 (39) | 0 | |
| No | 27 (61) | 91 (100) | |
| α-fetoprotein level (ng/dL) | 0.86 | ||
| < 20 | 19 (43) | 42 (46) | |
| > 20 | 25 (57) | 49 (54) | |
| Size (cm) | 0.03 | ||
| < 3 | 14 (32) | 30 (33) | |
| 3-5 | 21 (48) | 23 (25 | |
| > 5 | 9 (20) | 38 (42) | |
| Number of nodules | 0.51 | ||
| Single | 32 (73) | 65 (72) | |
| 2 HCC | 8 (18) | 12 (13) | |
| 3 HCC or more | 4 (9) | 14 (15) | |
| Macrovascular Invasion | 0.16 | ||
| No | 42 (95) | 73 (80) | |
| Yes | 2 (5) | 18 (20) | |
| Microvascular invasion | 0.24 | ||
| No | 29 (66) | 43 (47) | |
| Yes | 15 (34) | 48 (53) | |
| Type of hepatectomy | 0.58 | ||
| Wedge resection | 10 (23) | 16 (18) | |
| Segmentectomy | 26 (59) | 52 (57) | |
| More than 1 segment | 8 (18) | 23 (25) | |
ALT: Alanine transaminase; AST: Aspartate transaminase; HCC: Hepatocellular carcinoma.
Table 2.
Mortality and morbidity rates according to the presence/absence of portal hypertension
|
Portal hypertension |
P value | ||
| Yes (%) | No (%) | ||
| n | 44 | 91 | |
| 1 mo mortality | 2 (4.6) | 1 (1.1) | 0.20 |
| 3 mo mortality | 6 (13.6) | 3 (3.3) | 0.05 |
| Overall morbidity | 16 (37) | 29 (32) | 0.59 |
| Cardiac complications | 0 | 3 (3.3) | 0.20 |
| Pulmonary complications | 10 (23) | 18 (20) | 0.71 |
| Hepatic complications | 14 (32) | 12 (13) | 0.03 |
The 3-year and 5-year survival in patients without PH was higher than in patients with PH (68.4% and 61.2% vs 48.7% and 44.9% respectively, P = 0.047). These results are reported in Figure 1A.
Figure 1.
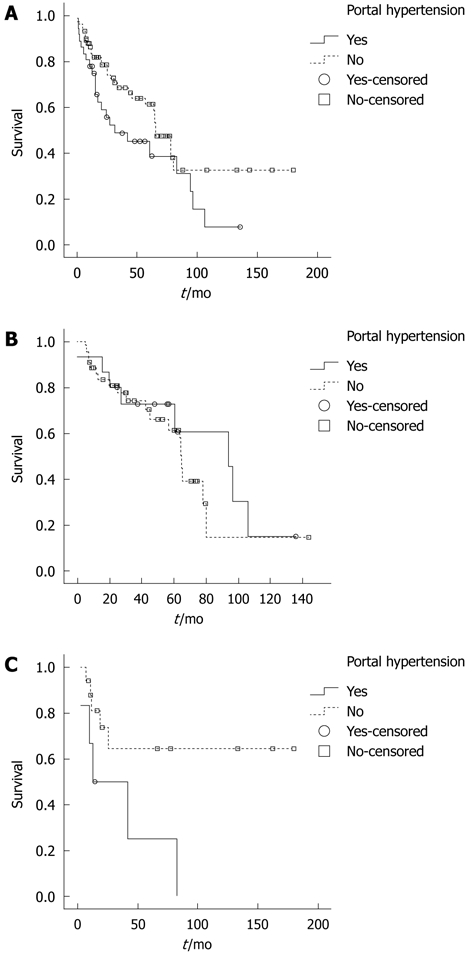
Overall survival analysis in patients. A: Overall survival analysis in patients with or without portal hypertension (PH); the difference between the two groups was statistically significant (P = 0.04); B: Overall survival analysis in Child-Pugh score (CP) A patients with or without PH, in the subgroup of patients submitted to limited resection (P = 0.45); C: Overall survival analysis in CP A patients with or without PH, in the subgroup of patients submitted to resection of 2 or more segments (P = 0.03).
Survival analysis in patients with Child-Pugh B cirrhosis did not demonstrate significant differences in patients with or without PH (3-year survival of 31.3% vs 11.9%, P = 0.465) (Table 3).
Table 3.
Survival analysis according to different groups of patient and presence/absence of portal hypertension
| Variable | n | Median survival (mo, 95% CI) |
Survival |
P value | |
| 3-yr | 5-yr | ||||
| Overall | 0.047 | ||||
| Without PH | 91 | 65.1 (49.7-80.4) | 68.4 | 61.2 | |
| With PH | 44 | 31.6 (3.4-59.9) | 48.7 | 44.9 | |
| Child-Pugh B | 0.465 | ||||
| Without PH | 10 | 27.7 (1.3-65.5) | 31.3 | 31.3 | |
| With PH | 15 | 15.1 (11.7-18.5) | 11.9 | - | |
| Child-Pugh A | 0.257 | ||||
| Without PH | 81 | 65.1 (47.7-82.4) | 72.0 | 63.2 | |
| With PH | 29 | 60.5 (6.4-114.6) | 63.0 | 57.3 | |
| CP A-limited Hx | 0.458 | ||||
| Without PH | 58 | 64.9 (62.9-67.0) | 72.4 | 61.4 | |
| With PH | 21 | 94.0 (54.0-134.0) | 72.7 | 72.7 | |
| CP A-Hx ≥ 2 segments | 0.035 | ||||
| Without PH | 23 | 163.9 (-) | 64.5 | 64.5 | |
| With PH | 8 | 64.4 (54.0-134.0) | 50.0 | 25.0 | |
CI: Confidence interval; PH: Portal hypertension; CP: Child-Pugh score; Hx: Hepatic resection.
Also, in Child-Pugh class A patients the survival analysis did not show significant differences in patients with or without PH, with a 3-year and 5-year survival of 63.0% and 57.3% vs 72.0% and 63.2% respectively (P = 0.257). These results are summarized in Table 3.
Furthermore, we evaluated the relationship between survival, extent of liver resection and PH in Child-Pugh class A patients.
In limited resections (wedge or one segment) we found no statistical differences between patients with or without PH. In these patients the 5-year survival was 72.4% and 61.4% respectively (P = 0.458, Figure 1B, Table 3).
When resection of two or more segments was performed, survival was significantly longer in patients without PH with a 5-year survival of 64.5% compared to 25.0% in patients with PH respectively (P = 0.035, Figure 1C, Table 3).
Multivariate analysis with Cox’s regression model confirmed that Child-Pugh class was related to survival with a HR of 2.57 (P < 0.01), whereas PH and type of hepatectomy were not related to survival (Table 4).
Table 4.
Multivariate Cox’s regression model of variables related to survival
| Variable | HR | 95% CI | P value |
| Child-Pugh class (B vs A) | 2.57 | 1.31-5.01 | 0.005 |
| Type of hepatectomy (≥ 2 segments vs limited) | 1.05 | 0.50-2.21 | 0.880 |
| PH (presence vs absence) | 1.51 | 0.84-2.68 | 0.160 |
CI: Confidence interval; PH: Portal hypertension.
DISCUSSION
Liver resection is currently the treatment of choice for single HCC and it is a safe treatment in terms of peri-operative complications, even in patients with liver cirrhosis[6,27]. The outcome of surgical resection is strongly related to hepatic functional reserve. For this reason, the majority of patients with liver cirrhosis cannot undergo surgery because of the high risk of postoperative liver failure.
PH in cirrhotic patients is considered a relative contraindication for surgery in EASL/AASLD guidelines. Bruix et al[12] analyzed the outcome of 29 Child-Pugh class A patients with PH [defined as porto-hepatic gradient (HVPG) greater than 10 mm Hg] and observed a higher likelihood of postoperative liver failure in these patients compared to those without PH. The authors justified these results because liver resection in patients with PH can reduce the portal vascular bed without a reduction of portal flow and this condition can lead to a further increase of portal pressure. These hypotheses were not confirmed by a study by Fujisaki et al[28] that reported on a group of 54 patients, in whom severity of PH was not worsened by liver resection. Llovet et al[29] in another study showed that clinical PH (assessed by the simultaneous presence of gastric-esophageal varices, thrombocytopenia lower than 100 000/mm3 and splenomegaly, or on a portal-hepatic venous pressure gradient greater than 10 mm Hg) is a predictor of postoperative liver failure and that it is related to long term survival. These authors studied 77 patients divided into 3 groups based on the presence or absence of PH and bilirubin level. The 5-year survival of patients without PH was 74%, while the patients with PH and bilirubin lower than 1 mg/dL had a 5-year survival of 50%, and the patients with PH and bilirubin greater than 1 mg/dL had a 5-year survival of 25%. Other studies confirmed a correlation between PH and increased mortality and complications. Poon et al[30], in a study of 1222 patients undergoing liver resection for hepato-biliary cancers, showed that the presence of thrombocytopenia at the time of surgery is a risk factor in multivariate analysis for surgical complications. Similarly, Jarnagin et al[31] in a study of 1803 patients showed that preoperative thrombocytopenia is associated with a postoperative increased risk of mortality.
On the contrary, other authors did not detect a significant correlation between PH and liver failure after surgery[32,33]. Ishizawa et al[23] analyzed 322 Child-Pugh class A patients and found good long term results in patients with or without PH, (3- and 5-year survival of 71% and 56% vs 81% and 71% respectively, P = 0.008). Capussotti et al[24], in a study of 217 patients including 99 with PH at the time of surgery, showed that the 3- and 5-year survival rates are greater in patients without the presence of PH (62% and 40% vs 45% and 29% respectively, P = 0.020). However, resection in patients with PH and good hepatic function (Child-Pugh A) had similar results in terms of 3- and 5-year survival (65% and 41% vs 60% and 41%, P = 0.503). This study also shows that patients with PH have a higher incidence of postoperative complications, particularly those related to the deterioration of liver function (27% vs 15%, P = 0.030). Kawano et al[25] evaluated the results of liver resection in patients with esophageal varices. This study found that patients with esophageal varices had a better 5-year survival (70% vs 47%, P = 0.045); the authors underlined that patients with PH had a more frequent early diagnosis of HCC due to more careful follow up. More recently, Choi et al[34] reported that the 5-year survival rate of patients with clinical PH affected by single nodular HCC without macrovascular invasion was 78.4%, even if in Child-Pugh A cirrhotic patients, the presence of clinically significant PH was significantly associated with postoperative hepatic failure and poor prognosis after resection of HCC.
The data from our study confirm the recent experiences in the literature. Patients with PH at the time of surgery showed worse liver function and this justifies the increased number of complications related to the deterioration of liver function and the increased postoperative 3-mo mortality. Long term survival was significantly related to PH with significantly shorter survival (P < 0.04).
Among different Child-Pugh class patients we did not observe statistically significant differences in 3- and 5-year survival between patients with or without PH. In Child-Pugh class A patients submitted to minor resection, survival was not significantly affected by PH. Our study is the first in the literature to demonstrate the relationship between survival, PH and extent of hepatectomy. Surgery can produce good long term survival in patients with PH submitted to limited resection; conversely, PH had strong adverse prognostic significance in patients who underwent resection of two or more segments. This data can help in the selection of patients and improve safety and long term results of surgery, as well as identify a group of patients who require resection of 2 or more segments, in whom resection is contraindicated and other non-surgical therapies should be applied.
This is a retrospective analysis from a single center. This allowed homogeneous data; however, multi-center studies are needed to confirm these results to reduce technical bias.
Our study confirms that the presence of PH at the time of surgery is not an absolute contraindication to resection in patients with liver cirrhosis. Although the rate of postoperative complications in patients with PH is greater, the results in terms of survival in the group of Child-Pugh class A patients is similar in patients without PH. Also, in patients with PH, limited liver resection can be performed with results comparable to those in patients without PH. Conversely, surgical resection of 2 or more segments in patients with PH results in significantly shorter survival and should not be recommended.
COMMENTS
Background
The presence of liver cirrhosis is the most important risk factor for the development of hepatocellular carcinoma (HCC): 85%-95% of HCCs arise in cirrhotic livers. Portal hypertension (PH) is related to increase in intrahepatic resistance due to the structural subversion of the cirrhotic liver and loss of vascular bed, and bleeding from gastro-esophageal varices is one of the most important complications of cirrhosis. The European Association for the Study of the Liver/American Association for the Study of Liver Diseases (AASLD) guidelines consider PH as a relative contraindication to liver resection, because of the high risk of postoperative liver failure, as reported in some clinical series. On the contrary, other authors have not detected a significant correlation between PH and liver failure after surgery.
Research frontiers
Surgical treatment is the most effective treatment for HCC and the mortality after surgery has decreased in recent years in relation to improved surgical techniques and peri-operative management of patients. Moreover, only about 30% of patients affected by HCC can be submitted to surgery, because of the characteristics of the tumor (stage, number of nodules, size, presence of vascular invasion), the general condition of the patient, or liver functional impairment, such as PH.
Innovations and breakthroughs
Recently, authors have not detected a significant correlation between PH and liver failure after surgery. The data from this study confirm the recent experiences in the literature. Survival analysis in patients with Child-Pugh A and B cirrhosis did not demonstrate significant differences in patients with or without PH. Moreover, limited resections (wedge or one segment) showed no statistical differences between patients with or without PH. When resection of two or more segments was performed, survival was significantly longer in patients without PH. Multivariate analysis with Cox’s regression model confirmed that Child-Pugh, but not PH and type of hepatectomy class, was related to survival. This study is the first in the literature to demonstrate the relationship between survival, PH and extent of hepatectomy. Surgery can result in good long term survival in patients with PH submitted to limited resection; conversely, PH had strong adverse prognostic significance in patients who underwent resection of two or more segments.
Applications
This study confirms that the presence of PH at the time of surgery is not an absolute contraindication to resection in patients with liver cirrhosis. Results in terms of survival in the group of Child-Pugh class A patients are similar to patients without PH. Furthermore, in patients with PH, limited liver resection can be performed with results comparable to those in patients without PH. Conversely, surgical resection of 2 or more segments in patients with PH resulted in significantly shorter survival and is not to be recommended.
Terminology
Clinical PH is defined according to AASLD guidelines as the presence of esophageal varices or thrombocytopenia (platelet count < 100 000/mm3), associated with splenomegaly.
Peer review
In this retrospective study, the authors compare the results of liver resection for HCC in patients with and without PH. The authors show that resection is safe in CTP. A patients regardless of PH, in patients undergoing limited resection. The authors show no difference in survival between those with and without PH in CTP A/B patients.
Footnotes
Peer reviewers: Chun-Qing Zhang, Professor, Department of Gastroenterology, Shandong Provincial Hospital, Jinan 250021, Shandong Province, China; Faisal M Sanai, Assistant Professor, Hepatobiliary Sciences, King Abdulaziz Medical City, PO Box 22490, Riyadh 11462, Saudi Arabia
S- Editor Wu X L- Editor Logan S E- Editor Zheng XM
References
- 1.Motola-Kuba D, Zamora-Valdés D, Uribe M, Méndez-Sánchez N. Hepatocellular carcinoma. An overview. Ann Hepatol. 2006;5:16–24. [PubMed] [Google Scholar]
- 2.Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 3.McGlynn KA, London WT. Epidemiology and natural history of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2005;19:3–23. doi: 10.1016/j.bpg.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Belghiti J, Kianmanesh R. Surgical treatment of hepatocellular carcinoma. HPB (Oxford) 2005;7:42–49. doi: 10.1080/13651820410024067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasaki A, Iwashita Y, Shibata K, Matsumoto T, Ohta M, Kitano S. Improved long-term survival after liver resection for hepatocellular carcinoma in the modern era: retrospective study from HCV-endemic areas. World J Surg. 2006;30:1567–1578. doi: 10.1007/s00268-005-0249-9. [DOI] [PubMed] [Google Scholar]
- 6.Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–330. doi: 10.1097/00000658-199903000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 8.Adachi E, Maehara S, Tsujita E, Taguchi K, Aishima S, Rikimaru T, Yamashita Y, Tanaka S. Clinicopathologic risk factors for recurrence after a curative hepatic resection for hepatocellular carcinoma. Surgery. 2002;131:S148–S152. doi: 10.1067/msy.2002.119496. [DOI] [PubMed] [Google Scholar]
- 9.Shah SA, Greig PD, Gallinger S, Cattral MS, Dixon E, Kim RD, Taylor BR, Grant DR, Vollmer CM. Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J Am Coll Surg. 2006;202:275–283. doi: 10.1016/j.jamcollsurg.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, Langer B, Grant DR, Greig PD, Gallinger S. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141:330–339. doi: 10.1016/j.surg.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 11.Wu CC, Cheng SB, Ho WM, Chen JT, Liu TJ, P’eng FK. Liver resection for hepatocellular carcinoma in patients with cirrhosis. Br J Surg. 2005;92:348–355. doi: 10.1002/bjs.4838. [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, Visa J, Bru C, Rodés J. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–1022. doi: 10.1016/s0016-5085(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 13.Ruzzenente A, Capra F, Pachera S, Iacono C, Piccirillo G, Lunardi M, Pistoso S, Valdegamberi A, D’Onofrio M, Guglielmi A. Is liver resection justified in advanced hepatocellular carcinoma? Results of an observational study in 464 patients. J Gastrointest Surg. 2009;13:1313–1320. doi: 10.1007/s11605-009-0903-x. [DOI] [PubMed] [Google Scholar]
- 14.Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002;122:1609–1619. doi: 10.1053/gast.2002.33411. [DOI] [PubMed] [Google Scholar]
- 15.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20–S37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Akridis EA, Llovet JM, Efremidis SC, Shouval D, Canelo R, Ringe B, Meyers WC. Hepatocellular carcinoma. Br J Surg. 1998;85:1319–1331. doi: 10.1046/j.1365-2168.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 18.Sanyal AJ, Bosch J, Blei A, Arroyo V. Portal hypertension and its complications. Gastroenterology. 2008;134:1715–1728. doi: 10.1053/j.gastro.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Chalasani N, Imperiale TF, Ismail A, Sood G, Carey M, Wilcox CM, Madichetty H, Kwo PY, Boyer TD. Predictors of large esophageal varices in patients with cirrhosis. Am J Gastroenterol. 1999;94:3285–3291. doi: 10.1111/j.1572-0241.1999.1539_a.x. [DOI] [PubMed] [Google Scholar]
- 20.Qamar AA, Grace ND, Groszmann RJ, Garcia-Tsao G, Bosch J, Burroughs AK, Ripoll C, Maurer R, Planas R, Escorsell A, et al. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol. 2009;7:689–695. doi: 10.1016/j.cgh.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 22.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 23.Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908–1916. doi: 10.1053/j.gastro.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 24.Capussotti L, Ferrero A, Viganò L, Muratore A, Polastri R, Bouzari H. Portal hypertension: contraindication to liver surgery? World J Surg. 2006;30:992–999. doi: 10.1007/s00268-005-0524-9. [DOI] [PubMed] [Google Scholar]
- 25.Kawano Y, Sasaki A, Kai S, Endo Y, Iwaki K, Uchida H, Shibata K, Ohta M, Kitano S. Short- and long-term outcomes after hepatic resection for hepatocellular carcinoma with concomitant esophageal varices in patients with cirrhosis. Ann Surg Oncol. 2008;15:1670–1676. doi: 10.1245/s10434-008-9880-7. [DOI] [PubMed] [Google Scholar]
- 26.Belghiti J, Clavien P, Gadzijev E, Garden JO, Lau W, Makuuchi M, Strong RW. The Brisbane 2000 terminology of liver anatomy and resections. HPB. 2000;2:333–339. [Google Scholar]
- 27.Torzilli G, Makuuchi M, Inoue K, Takayama T, Sakamoto Y, Sugawara Y, Kubota K, Zucchi A. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg. 1999;134:984–992. doi: 10.1001/archsurg.134.9.984. [DOI] [PubMed] [Google Scholar]
- 28.Fujisaki S, Miyake H, Amano S, Nakayama H, Tomita R, Fukuzawa M. Influence of the extent of hepatectomy on the portal hypertensive state in patients with hepatoma. Hepatogastroenterology. 1999;46:2490–2494. [PubMed] [Google Scholar]
- 29.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 30.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708; discussion 708-710. doi: 10.1097/01.sla.0000141195.66155.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406; discussion 406-407. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, Takayama T, Makuuchi M. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206; discussion 1206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 33.Capussotti L, Ferrero A, Viganò L, Polastri R, Tabone M. Liver resection for HCC with cirrhosis: surgical perspectives out of EASL/AASLD guidelines. Eur J Surg Oncol. 2009;35:11–15. doi: 10.1016/j.ejso.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Choi GH, Park JY, Hwang HK, Kim DH, Kang CM, Choi JS, Park YN, Kim do Y, Ahn SH, Han KH, et al. Predictive factors for long-term survival in patients with clinically significant portal hypertension following resection of hepatocellular carcinoma. Liver Int. 2011;31:485–493. doi: 10.1111/j.1478-3231.2010.02436.x. [DOI] [PubMed] [Google Scholar]


