ABSTRACT
BACKGROUND
In the United States, mortality from cardiovascular disease has become increasingly common among HIV-infected persons. One-third of HIV-infected persons in care may rely on state-run AIDS Drug Assistance Programs (ADAPs) for cardiovascular disease-related prescription drugs. There is no federal mandate regarding ADAP coverage for non-HIV medications.
OBJECTIVE
To assess the consistency of ADAP coverage for type 2 diabetes, hypertension, hyperlipidemia, and smoking cessation using clinical guidelines as the standard of care.
DESIGN
Cross-sectional survey of 53 state and territorial ADAP formularies.
MAIN MEASURES
ADAPs covering all first-line drugs for a cardiovascular risk factor were categorized as “consistent” with guidelines, while ADAPs covering at least one first-line drug, but not all, for a cardiovascular risk factor, were categorized as “partially consistent”. ADAPs without coverage were categorized as “no coverage”.
KEY RESULTS
Of 53 ADAPs, four (7.5%) provided coverage consistent with guidelines (coverage for all first-line drugs) for all four cardiovascular risk factors. Thirteen (24.5%) provided no coverage for all four risk factors. Thirty-six (68%) provided at least partially consistent coverage for at least one surveyed risk factor. State ADAPs provided coverage consistent with guidelines most frequently for type 2 diabetes (28%), followed by hypertension (25%), hyperlipidemia (15%) and smoking cessation (8%). Statins (66%) were most commonly covered and nicotine replacement therapies (9%) least often. Many ADAPs provided no first-line treatment coverage for hypertension (60%), type 2 diabetes (51%), smoking cessation (45%), and hyperlipidemia (32%).
CONCLUSIONS
Consistency of ADAP coverage with guidelines for the surveyed cardiovascular risk factors varies widely. Given the increasing lifespan of HIV-infected persons and restricted ADAP budgets, we recommend ADAP coverage be consistent with guidelines for cardiovascular risk factors.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-011-1807-5) contains supplementary material, which is available to authorized users.
KEY WORDS: AIDS, HIV, public assistance, AIDS drug assistance program, cardiovascular disease
In the third decade since the recognition of human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS), HIV-infected individuals are living longer due, in large part, to potent antiretroviral medications.1,2 Persons aged 50 years and older now account for nearly one-quarter of individuals with HIV/AIDS in the United States.3 As a result, mortality in HIV-infected persons is increasingly the result of non-AIDS-related causes such as cardiovascular disease.4–6 A recent study estimated that approximately 10% of deaths among HIV-infected persons are due to cardiovascular disease, of which, type 2 diabetes, hypertension, hyperlipidemia and tobacco use are well-recognized and treatable cardiovascular-associated conditions.4 Persons with HIV are more likely to smoke compared to their HIV-uninfected counterparts.7 Moreover, certain classes of antiretrovirals contribute to increased risk of type 2 diabetes, hyperlipidemia, and possibly cardiovascular disease.8–11
Approximately one-third of HIV-infected persons in care, an estimated 183,000 individuals, are enrolled in state AIDS Drug Assistance Programs (ADAPs).12 Almost three-quarters of ADAP patients are uninsured and rely solely on ADAPs for their prescription drugs.13 Some ADAPs also provide health insurance coverage and cover out-of-pocket health care expenses.13 ADAPs are funded largely by the Health Resources and Service Administration’s HIV/AIDS Bureau through Part B of the Ryan White HIV/AIDS Program and, to a smaller extent, by the states themselves. Each state ADAP, however, is primarily responsible for determining the composition of its formulary.12 ADAPs are only required to include one drug from each antiretroviral class and are not mandated to cover non-HIV medications.12–14 Over the last few years, due in part to the economic recession and increasing ADAP client enrollment, ADAPs in certain states have taken cost-containment measures, including restricting their formularies.13,15
Given the increasing proportion of deaths attributable to cardiovascular disease among HIV-infected persons and the absence of mandates or guidelines informing coverage, we were interested in the degree of variation in state ADAP prescription drug coverage for four modifiable cardiovascular risk factors—type 2 diabetes, hypertension, hyperlipidemia, and tobacco use. We sought to determine whether ADAP coverage was congruent with current clinical guidelines for each of these four conditions.
METHODS
We systematically surveyed ADAP formularies from the 50 states, Washington, D.C., the Commonwealth of Puerto Rico and the U.S. Virgin Islands (which from here on we will refer to as “states”) from August 1 to September 15, 2010. First, we accessed formularies on each state department of health’s or related website. Subsequently, each state’s health department was contacted directly (either by e-mail or by telephone if there was no response to e-mail) to confirm the date of the most current formulary. If the website’s formulary was out-of-date or unavailable, we requested the most up-to-date formulary be sent via e-mail or fax (Appendix available online). Dates for ADAP formularies ranged from January 2008 to August 2010.
We documented the presence or absence of medications on each formulary for treating type 2 diabetes, hypertension, hyperlipidemia, and tobacco use. We used clinical guidelines as the reference standard of care since their use is intended to improve quality of care, allocate resources efficiently, and avoid unnecessary variation in clinical practice.16,17 For each state ADAP, we assessed the level of prescription drug coverage of first-line medications for each cardiovascular risk factor as follows: “consistent” corresponded to drug coverage which was congruent with current guidelines, “partially consistent” corresponded to drug coverage which was not in full agreement with guidelines, and “no coverage” signified no drug coverage by the state ADAP. If a state ADAP covered non-preferred drugs in addition to recommended drugs, only the recommended first-line drugs were considered when defining the level of coverage, as the focus was on documenting congruence with the clinical guidelines.
Type 2 Diabetes
Persons with type 2 diabetes may require oral hypoglycemic agents and/or insulin. Metformin plus a sulfonylurea or metformin plus basal insulin is recommended as an appropriate first step, as clinically indicated, according to the Medical Management of Hyperglycemia in Type 2 Diabetes: A Consensus Algorithm for the Initiation and Adjustment of Therapy from the American Diabetes Association and the European Association for the Study of Diabetes.18
We defined a state ADAP formulary’s level of prescription drug coverage for type 2 diabetes as follows: consistent (metformin, a sulfonylurea, and basal insulin covered), partially consistent (at least one of the aforementioned classes covered, but not all), or no coverage (no first-line glucose-lowering medications covered).
We also documented coverage for thiazolidinediones, a frequently prescribed class of oral hypoglycemics, which is not recommended as first-line treatment.18
Hypertension
Individuals with hypertension often require at least two medications for optimal blood pressure control.19,20 Based on the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7), appropriate therapy for primary hypertension includes a thiazide diuretic plus a beta-blocker, calcium-channel blocker, ACE-inhibitor, or angiotensin-II receptor blocker, if clinically indicated.19 JNC7 also states that ACE-inhibitors or angiotensin-II receptor blockers can be used interchangeably.
We defined a state ADAP formulary’s level of prescription drug coverage for hypertension as follows: consistent (thiazide diuretic, ACE-inhibitor or angiotensin-II receptor blocker, beta-blocker, and calcium-channel blocker covered), partially consistent (at least one of the aforementioned classes covered, but not all), or no coverage (no first-line anti-hypertensive medications covered).
Hyperlipidemia
For persons with hyperlipidemia, HMG-CoA reductase inhibitors (statins) are used either alone or in combination with nicotinic acid and/or fibrates. The 3rd Report for the National Cholesterol Education Program for the Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (NCEP) recommends four medication classes: statins as first-line treatment with bile acid sequestrants, nicotinic acid, and/or fibrates, if clinically indicated.21
We categorized level of prescription drug coverage for hyperlipidemia as follows: consistent (statins, bile acid sequestrants, nicotinic acid, and fibrates covered), partially consistent (at least one of the aforementioned classes covered, but not all), and no coverage (no first-line lipid-lowering medications covered).
We also documented coverage for ezetimibe, a commonly prescribed lipid-lowering medication, which is not included in the NCEP guidelines.21
Smoking Cessation
Counseling and medication in combination for tobacco use and dependence are recommended for smoking cessation.22 Additionally, dual pharmacotherapy may be more effective than one method alone.22 Treating Tobacco Use and Dependence: 2008 update U.S. Public Health Service Clinical Practice Guideline executive summary recommends the following medications as effective for long-term smoking cessation: five nicotine-based methods (gum, inhaler, lozenges, spray, patch) and two non-nicotine therapies (bupropion sustained release, varenicline).22
We classified level of prescription drug coverage for smoking cessation as follows: consistent (any type of nicotine-replacement therapy, bupropion, and varenicline covered), partially consistent (at least one of the aforementioned classes covered, but not all), and no coverage (no first-line smoking cessation therapy covered). Since bupropion is used for smoking cessation and depression treatment, we assumed coverage of bupropion for smoking cessation if a state ADAP formulary did not explicitly state that bupropion could not be prescribed for smoking cessation.
RESULTS
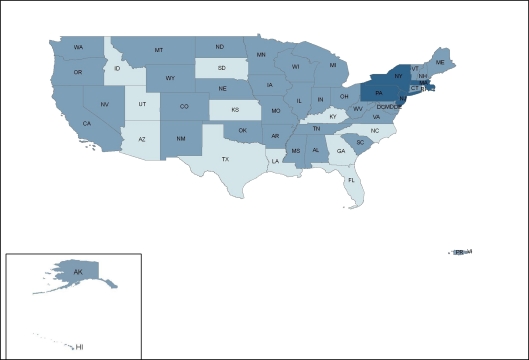
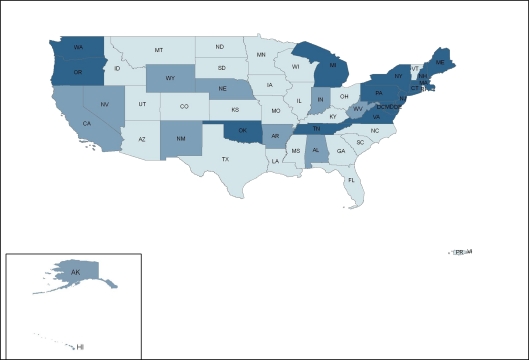
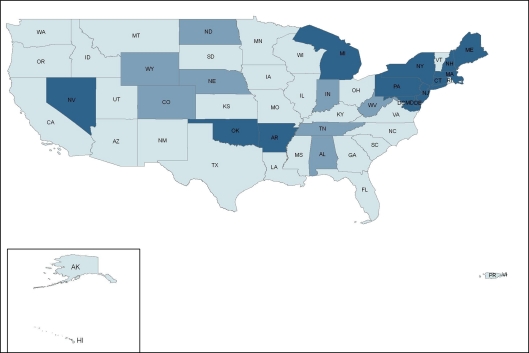
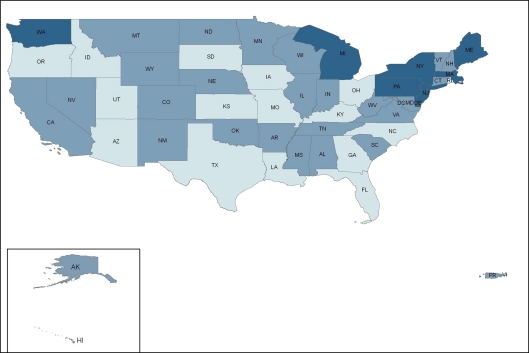
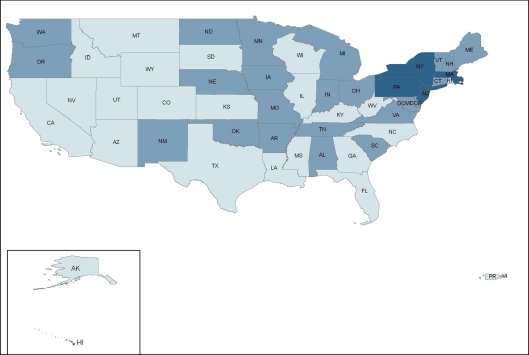
ADAP prescription drug coverage for type 2 diabetes, hypertension, hyperlipidemia, and smoking cessation varies considerably by state. In a survey of ADAP formularies from the 50 U.S. states, Washington, D.C., and two territories, 4 states (7.5%)—Massachusetts, New Jersey, New York, and Pennsylvania—provided prescription drug coverage consistent with clinical guidelines (coverage for all first-line drugs) for all four cardiovascular risk factors; 36 states (68%) provided partially consistent coverage for at least one cardiovascular risk factor; and 13 states (24.5%) offered no coverage (Fig. 1). State ADAP formularies covered treatment consistent with clinical guidelines most frequently for type 2 diabetes (28%), followed by hypertension (25%), hyperlipidemia (15%), and smoking cessation (8%) (Fig. 2, 3, 4, 5).
Figure 1.
Consistency of state ADAP prescription drug coverage with clinical guidelines for the treatment of modifiable cardiovascular risk factors.  No coverage for any risk factor,
No coverage for any risk factor,  At least partially consistent coverage for at least one risk factor,
At least partially consistent coverage for at least one risk factor,  Consistent coverage for all four risk factors.
Consistent coverage for all four risk factors.
Figure 2.
State variation in ADAP prescription drug coverage for diabetes mellitus.  No coverage,
No coverage,  Partially consistent with clinical guidelines,
Partially consistent with clinical guidelines,  Consistent with clinical guidelines.
Consistent with clinical guidelines.
Figure 3.
State variation in ADAP prescription drug coverage for hypertension.  No coverage,
No coverage,  Partially consistent with clinical guidelines,
Partially consistent with clinical guidelines,  Consistent with clinical guidelines.
Consistent with clinical guidelines.
Figure 4.
State variation in ADAP prescription drug coverage for hyperlipidemia.  No coverage,
No coverage,  Partially consistent with clinical guidelines,
Partially consistent with clinical guidelines,  Consistent with clinical guidelines.
Consistent with clinical guidelines.
Figure 5.
State variation in ADAP prescription drug coverage for smoking cessation.  No coverage,
No coverage,  Partially consistent with clinical guidelines,
Partially consistent with clinical guidelines,  Consistent with clinical guidelines.
Consistent with clinical guidelines.
Type 2 Diabetes
Fifteen states (28%) provided prescription drug coverage consistent with guidelines, 11 states (21%) offered coverage partially consistent with guidelines, and 27 states (51%) provided no coverage for first-line diabetic medications (Fig. 2). The most commonly offered medication for type 2 diabetes on ADAP formularies was metformin (49%), followed by sulfonylureas (42%), thiazolidinediones (34%), and insulin (28%). States categorized as partially consistent with guidelines most frequently covered both metformin and sulfonylureas but did not cover insulin. Of note, thiazolidinediones, though offered by ten ADAPs, are not recommended as first-line treatment for type 2 diabetes according to current guidelines.18
Hypertension
Thirteen states (25%) offered prescription drug coverage consistent with guidelines for hypertension, eight states (15%) provided coverage partially consistent with guidelines, and 32 states (60%) offered no coverage for recommended anti-hypertensive medications (Fig. 3). ACE-inhibitors (38%) were covered most often, followed by thiazides (32%), beta-blockers (30%), calcium-channel blockers (26%), and angiotensin-II receptor blockers (26%). Among states with coverage partially consistent with guidelines, ACE-inhibitors were most often covered either alone or in combination with thiazides or beta-blockers.
Hyperlipidemia
Eight state ADAPs (15%) had formularies that were consistent with guidelines for hyperlipidemia, while 28 states (53%) provided coverage partially consistent with guidelines, and 17 states (32%) provided no coverage of first-line treatments for hyperlipidemia (Fig. 4). Among lipid-lowering prescription drugs, statins (66%) were most often covered, followed by fibrates (62%), niacin (32 %) and bile acid sequestrants (17%). States with coverage partially consistent with guidelines most frequently covered both statins and fibrates. Of note, eleven ADAPs covered ezetimibe, which is not recommended by clinical guidelines.21
Smoking Cessation
Four state ADAPs (8%) provided prescription drug coverage consistent with guidelines, 25 states (47%) offered coverage partially consistent with guidelines, and 24 states (45%) provided no coverage for recommended treatments for smoking cessation (Fig. 5). The most commonly covered medication for smoking cessation was bupropion (53%). Varenicline (13%) and nicotine replacement therapies (9%) were covered less often. Bupropion was the most frequently covered prescription drug in states with coverage partially consistent with guidelines.
DISCUSSION
Wide variation by state exists in ADAP prescription drug coverage for the management of type 2 diabetes, hypertension, hyperlipidemia, and tobacco use. In our systematic survey of ADAP formularies, we identified only four states that provided prescription drug coverage consistent with clinical practice guidelines (coverage for all first-line drugs) for all four modifiable cardiovascular risk factors. Thirteen states provided no coverage for the four surveyed conditions. ADAPs most often provided coverage consistent with guidelines for type 2 diabetes and least often for smoking cessation. ADAPs more often provided absolutely no first-line drug coverage for type 2 diabetes and hypertension compared with the other two conditions. Prescription drug coverage was partially consistent with clinical guidelines most commonly for hyperlipidemia and smoking cessation. Of all medication classes, statins were most often covered and nicotine replacement therapies least often. The reasons for variation in coverage for these four conditions are most likely multifactorial, including ADAP’s historical context, funding mechanism, diverse approaches to implementation, and complexities of formulary design.
The historical context out of which ADAPs developed may contribute to state differences in prescription drug coverage for cardiovascular risk factors. ADAPs began as “AZT Assistance Programs” in 1987 with the sole purpose of providing HIV drug coverage at a time when HIV was associated with a rapid clinical course and high mortality.23 Since the 2006 reauthorization of the Ryan White Program, ADAPs have been mandated to provide, at minimum, one medication in each HIV-medication class but are not required to provide any additional medications.23 A prior survey of ADAP formularies indicated that less than half of ADAPs provide hepatitis C treatment and that coverage for opportunistic infections varies.13 Although, historically, states with the highest number of AIDS cases have received a greater proportion of federal funding, the number of HIV/AIDS cases does not appear to explain the variability in ADAP prescription drug coverage. Among the ten state ADAPs with highest cumulative number of HIV/AIDS cases, three provided coverage consistent with guidelines for all four conditions, while four provided absolutely no coverage.24,25
Disparities in prescription drug coverage may stem from financial constraints facing ADAPs. While ADAP client enrollment has continued to increase, federal funding has reached a plateau, and state funding has declined substantially due, in large part, to the economic recession.13 Consequently, some ADAPs have taken cost-containment measures, such as implementing waiting lists or restricting prescription drug formularies.13,15 Despite non-HIV medications accounting for less than 10% of the prescription drug budget, several states have removed non-HIV prescription drugs from their formularies.13,15 Expanded HIV testing and changes in eligibility for HIV treatment will contribute to ADAPs rising caseload and expenditures.26–28
Differences in ADAP formularies may also be explained by the diverse approaches used by ADAPs to provide prescription drug coverage. ADAPs are considered “a payer of last resort” and are intended to provide a “safety net” for individuals who are uninsured or underinsured.13,23,29 Clients may have prescription drug coverage through programs such as Medicaid or Medicare Part D, which may complement state ADAP formularies.13 However, state ADAPs with restrictive criteria for eligibility and limited prescription drug coverage tend to exist in states with Medicaid programs with equally stringent enrollment criteria and minimal drug coverage.30 ADAPs may also purchase health insurance for clients which provides access to a particular insurance plan’s formulary and may offer short term prescription drug coverage for clients until ADAP enrollment.13,15 In these cases, ADAP formularies would not be expected to be particularly comprehensive. For medications not offered on ADAP formularies, clients may rely on pharmaceutical assistance programs which are limited in scope and have complex and time-consuming application processes.31 Lastly, state ADAPs may structure their formularies to offer a wide array of medications. For example, the four ADAPs (MA, NJ, NY, PA) that provide coverage consistent with clinical guidelines have created comprehensive formularies (Appendix available online).
Variations may reflect a delay in adapting to clinical guidelines or even a lack of awareness about guidelines by each state’s ADAP advisory committee, which determines the composition of the formulary. For instance, thiazolidinediones, a relatively more expensive, second-tier class of type 2 diabetes treatment, were covered more frequently by ADAPs than insulin.18,32 The coverage of thiazolidinediones may reflect their use for the treatment of antiretroviral-associated lipoatrophy, though clinical trial results have been inconsistent.33,34 More concerning is that rosiglitazone, a thiazolidinedione associated with increased cardiovascular risk, was available on eleven formularies.35–37 Similarly, ezetimibe, a costly lipid-lowering medication without proven clinical benefit and with possible increased cardiovascular risk, was also covered on several ADAP formularies.38,39 In spite of high smoking rates among HIV-infected persons, only four states provided coverage consistent with clinical guidelines for smoking cessation.7 Though ADAPs frequently covered bupropion, it was usually included for depression treatment not smoking cessation.
In light of the economic constraints facing state ADAPs and the increasing numbers of persons requiring treatment for HIV/AIDS and chronic conditions, ADAPs may decrease expenditures with coverage of effective prescription drugs and additional price reductions of prescription drugs. Currently, among HIV-infected persons, the effect of drug coverage for cardiovascular risk factors on clinical outcomes and costs is unknown. However, covering effective treatment for the surveyed risk factors may be more cost-effective over time given the increasing longevity of HIV-infected persons. Additionally, most of the recommended medications are available as generics. ADAPs negotiate with pharmaceutical manufacturers and utilize the 340B pharmacy program for access to discounted prescription drugs.25 Further reductions in cost may be possible if drug discounts available to federal entities, such as the Department of Defense, were also available to all ADAPs.25,29
The most significant impact on ADAPs may occur with implementation of the Affordable Care Act (ACA). The expansion of Medicaid eligibility and provision of subsidies to low-income persons to purchase insurance will likely decrease the number of individuals requiring ADAPs, therefore lessening the burden on these programs. With cost-sharing between ADAPs and Medicare Part D during the Medicare coverage gap (or “doughnut hole”), ADAPs and individuals who depend on both programs will have decreased financial burden. Past and current economic hardships have shed light on our fragmented public health insurance system and its effect on persons who rely on ADAPs. The ACA may help to rectify this patchwork system in order to provide more comprehensive and stable health care coverage.
Our study has several limitations. First, we used clinical guidelines as standard of care to determine adequacy of ADAP drug coverage. Yet, there remains uncertainty regarding the impact of clinical guidelines on health outcomes and the efficacy of certain drugs in the prevention of cardiovascular disease.17 Second, the actual clinical impact of limited ADAP drug coverage for cardiovascular risk factors is unknown. Nonetheless, studies suggest that variation in the availability of needed prescription drugs can alter health outcomes, including life expectancy among HIV-infected persons.40 Third, as previously noted, some ADAP clients may have assistance from other programs which may provide prescription drug coverage for these co-morbidities. Lastly, although we verified the most updated formulary with each state ADAP, given the dynamic nature of ADAP formularies, it is possible that we did not have the most recent information for some states.
Our findings indicate that most ADAPs do not provide guideline-consistent prescription drug coverage for type 2 diabetes, hypertension, hyperlipidemia, or smoking cessation. Given that state ADAPs are under extreme financial constraints, we highlight this variation in coverage by state to bring attention to the challenges facing HIV-infected persons who have little or no access to certain prescription drugs. Policymakers should address the root causes of this variation, and, at minimum, in an effort to maximize value, provide a comprehensive ADAP formulary informed by clinical guidelines. Further research is needed to evaluate the factors associated with variation in prescription drug coverage, the potential effects of this variation, and the impact of reducing variation on health outcomes.
Electronic supplementary material
(DOC 58 kb)
Contributors
We would like to thank Laura Cheever, MD ScM, Ruth Finkelstein, ScD, and David Paltiel, PhD, for their critical review of an earlier version of this manuscript. We would also like to thank Russell Barbour, PhD, for assistance with our figures.
Funders: This work was funded by Robert Wood Johnson Foundation, Department of Veterans Affairs, National Institute of Mental Health, and Center for Interdisciplinary Research on AIDS. The funders had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Prior presentations: Center for Interdisciplinary Research on AIDS at Yale University, AIDS Science Day 2011, New Haven, Connecticut, March 23, 2011; Society of General Internal Medicine 34th Annual Meeting, Phoenix, AZ, May 4, 2011.
Conflict of Interest None disclosed.
Footnotes
Drs. Oni J. Blackstock and Karen H. Wang contributed equally to this manuscript
REFERENCES
- 1.Harrison KM, Song R, Zhang X. Life expectancy after HIV diagnosis based on national HIV surveillance data from 25 states, United States. J Acquir Immune Defic Syndr. 2010;53:124–130. doi: 10.1097/QAI.0b013e3181b563e7. [DOI] [PubMed] [Google Scholar]
- 2.Bhaskaran K, Hamouda O, Sannes M, et al. CASCADE Collaboration. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008;300:51–59. doi: 10.1001/jama.300.1.51. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention: Persons Aged 50 Years and Older. Available at: http://www.cdc.gov/hiv/topics/over50/index.htm. Accessed June 16, 2011.
- 4.Smith C, Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group Association between modifiable and non-modifiable risk factors and specific causes of death in the HAART era: The Data Collection on Adverse Events of Anti-HIV Drug Study. 16th Conference on Retroviruses and Opportunistic Infections; 2009 Feb 8–11; Montreal, Canada. Abstract 145.
- 5.Mocroft A, Reiss P, Gasiorowski J, et al. Serious fatal and non-fatal non-AIDS-defining illnesses in Europe. Paper presented at: 16th Conference on Retroviruses and Opportunistic Infections; 2009 Feb 8-11; Montreal, Canada. Abstract 707.
- 6.Crum NF, Riffenburgh RH, Wegner S, et al. Triservice AIDS Clinical Consortium. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41:194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 7.Tesoriero JM, Gieryic SM, Carrascal A, Lavigne HE. Smoking among HIV positive New Yorkers: prevalence, frequency, and opportunities for cessation. AIDS Behav. 2010;14:824–835. doi: 10.1007/s10461-008-9449-2. [DOI] [PubMed] [Google Scholar]
- 8.Ledergerber B, Furrer H, Rickenbach M. et al; Swiss HIV Cohort Study. Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clin Infect Dis. 2007;45:111–119. doi: 10.1086/518619. [DOI] [PubMed] [Google Scholar]
- 9.Kotler DP. HIV and antiretroviral therapy: lipid abnormalities and associated cardiovascular risk in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;49:S79–S85. doi: 10.1097/QAI.0b013e318186519c. [DOI] [PubMed] [Google Scholar]
- 10.Friis-Moller N, Sabin CA, Weber R, et al. Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 11.Friis-Moller N, Reiss P, Sabin CA, et al. Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 12.The Henry J. Kaiser Family Foundation: HIV/AIDS Policy Fact Sheet: AIDS Drug Assistance Program, April 2009. Available at: http://www.kff.org/hivaids/upload/1584_10.pdf. Accessed June 16, 2011.
- 13.National Alliance of State and Territorial Directors. National ADAP Monitoring Project Annual Report, May 2010. Available at: http://www.nastad.org/Docs/highlight/201053_2010%20National%20ADAP%20Monitoring%20Report.pdf. Accessed June 16, 2011.
- 14.Smith SR, Buchanan RJ. The AIDS drug assistance programs and coverage of HIV-related medications. Ann Pharmacother. 2001;35:155–166. doi: 10.1345/aph.10077. [DOI] [PubMed] [Google Scholar]
- 15.Bassett IV, Farel C, Szmuilowicz ED, Walensky RP. HIV/AIDS: AIDS Drug Assistance Programs in the era of routine HIV testing. Clin Infect Dis. 2008;47:695–701. doi: 10.1086/590936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39:II46–II54. doi: 10.1097/00005650-200108002-00003. [DOI] [PubMed] [Google Scholar]
- 17.Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J. Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318:527–530. doi: 10.1136/bmj.318.7182.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 20.Cushman WC, Ford CE, Einhorn PT, et al. ALLHAT Collaborative Research Group. Blood pressure control by drug group in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) J Clin Hypertens (Greenwich) 2008;10:751–760. doi: 10.1111/j.1751-7176.2008.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Cholesterol Education Program Third Report of the National Cholesterol Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Final Report. Available at: http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3_rpt.htm. Accessed June 16, 2011.
- 22.2008 PHS Guideline Update Panel, Liaisons, and Staff. Treating tobacco use and dependence: 2008 update U.S. Public Health Service Clinical Practice Guideline executive summary. Respir Care. 2008;53:1217–22. [PubMed]
- 23.National Alliance of State and Territorial AIDS Directors, National ADAP Monitoring Program Annual Report, April 2009. Available at: http://www.nastad.org/Docs/highlight/200946_7861%20FULL%20REPORT%20v3.pdf. Accessed June 16, 2011.
- 24.Centers for Disease Control and Prevention: HIV Surveillance Report, 2008; vol. 20. Available at: http://www.cdc.gov/hiv/surveillance/resources/reports/2008report/. Accessed June 16, 2011.
- 25.Kaiser Family Foundation. The HIV/AIDS Epidemic in the United States. Available at: http://www.kff.org/hivaids/upload/3029-11.pdf. Accessed June 16, 2011.
- 26.Branson BM, Handsfield HH, Lampe MA, et al. Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. [PubMed] [Google Scholar]
- 27.Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed June 16, 2011.
- 28.Thompson MA, Aberg JA, Cahn P, et al. International AIDS Society-USA. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304:321–333. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 29.Taylor J. National Health Policy Forum. Caring for “Ryan White”: The Fundamentals of HIV/AIDS Treatment Policy. Available at: http://www.nhpf.org/library/background-papers/BP_RyanWhite_08-22-05.pdf. Accessed June 16, 2011.
- 30.Finkelstein R. Ryan White Care Act Reauthorization 2005: Title I and Title II Health Services Expenditures Patterns. Examination of Fiscal Management and the Allocation of CARE Act Resources. New York Academy of Medicine: New York, 2005.
- 31.Choudhry NK, Lee JL, Agnew-Blais J, Corcoran C, Shrank WH. Drug company-sponsored patient assistance programs: a viable safety net? Health Aff (Millwood) 2009;28:827–834. doi: 10.1377/hlthaff.28.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drugs for Type 2 Diabetes: Treatment guidelines from the Medical Letter. 2008. Issue 71, p. 47. Available at: http://secure.medicalletter.org/TG-article-71a. Accessed June 16, 2011.
- 33.Carr A, Workman C, Carey D, et al. Rosey Investigators. No effect of rosiglitazone for treatment of HIV-1 lipoatrophy: randomised, double-blind, placebo-controlled trial. Lancet. 2004;363:429–438. doi: 10.1016/S0140-6736(04)15489-5. [DOI] [PubMed] [Google Scholar]
- 34.Slama L, Lanoy E, Valantin MA, et al. Effect of pioglitazone on HIV-1-related lipodystrophy: a randomized double-blind placebo-controlled trial (ANRS 113) Antivir Ther. 2008;13:67–76. [PubMed] [Google Scholar]
- 35.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 36.Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 37.Nissen SE, Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med. 2010;170:1191–1201. doi: 10.1001/archinternmed.2010.207. [DOI] [PubMed] [Google Scholar]
- 38.Drugs for Lipids. Treatment Guidelines from the Medical Letter. 2008. Issue 66, p. 9. Available at: http://secure.medicalletter.org/TG-article-66a. Accessed June 16, 2011.
- 39.Kastelein JJ, Akdim F, Stroes ES, et al. ENHANCE Investigators. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–1443. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 40.Johri M, Paltiel DA, Goldie SJ, Freedberg KA. State AIDS Drug Assistance Programs: equity and efficiency in an era of rapidly changing treatment standards. Med Care. 2002;40:429–441. doi: 10.1097/00005650-200205000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 58 kb)