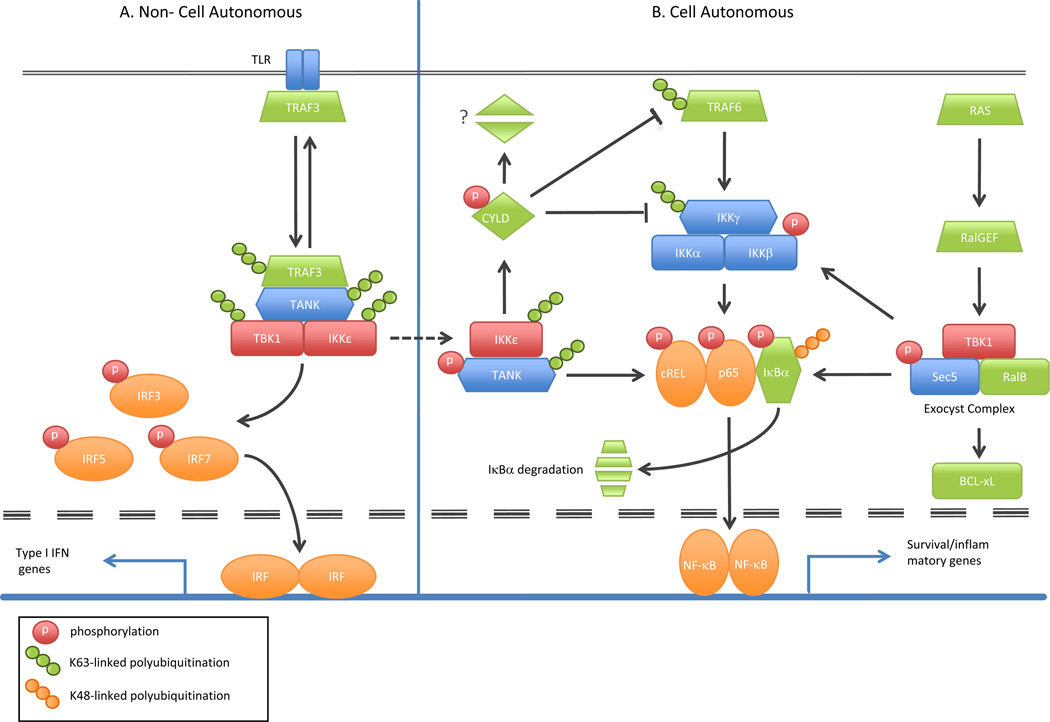
Figure 2. Cell autonomous and non-cell autonomous roles of IKKε and TBK1 in inflammation and cancer.
(A) Engagement of toll-like receptors (TLRs) initiates Lys-63 linked ubiquitination of TRAF3 and recruitment of IKKε and TBK1 through the adaptor molecule TANK. This recruitment promotes Lys-63 linked ubiquitination and activation of IKKε/TBK1. TANK is modified by both ubiquitination and phosphorylation through IKKε/TBK1, although it is unclear how these activities are related. The TANK/IKKε/TBK1 complex can then target interferon response factors (IRF3, IRF5, and IRF7) for phosphorylation and promote translocation to the nucleus where IFN target genes are activated. This cascade primarily defines the non-cell autonomous functions of the IKK-related kinases. In parallel to the interferon pathways, IKKε and TBK1 also phosphorylate many NF-κB effectors such as IκBα, p65 and cREL in response to cellular stimuli. This activity facilitates NF-κB nuclear translocation as a homo- or hetero- dimer and induces numerous inflammatory and survival- related target genes. (B) The IKKε/TBK1 oncogenic pathways function in a cell autonomous manner and are predominantly driven by aberrant levels of IKKε/TBK1. In this context, oncogenic RAS activates RalGEF, which in turn recruits the exocyst complex consisting of TBK1, RalB and Sec5, amongst other secretory proteins. TBK1 mediates phosphorylation of multiple effectors including Sec5, IKKβ, and IκBα that ultimately lead to the activation of both BCL-xL and NF-κB. Similarly, aberrant levels of IKKε in cancer also promote NF-κB activation through the phosphorylation of CYLD. CYLD negatively regulates NF-κB signaling by deubiquitinating Lys-63 linked ubiquitin chains on TRAF6 and IKKγ. It is hypothesized that IKKε-mediated phosphorylation of CYLD leads to either its degradation or sequestration, thereby inactivating its negative role on the NF-kB signaling cascade.