Figure 3.
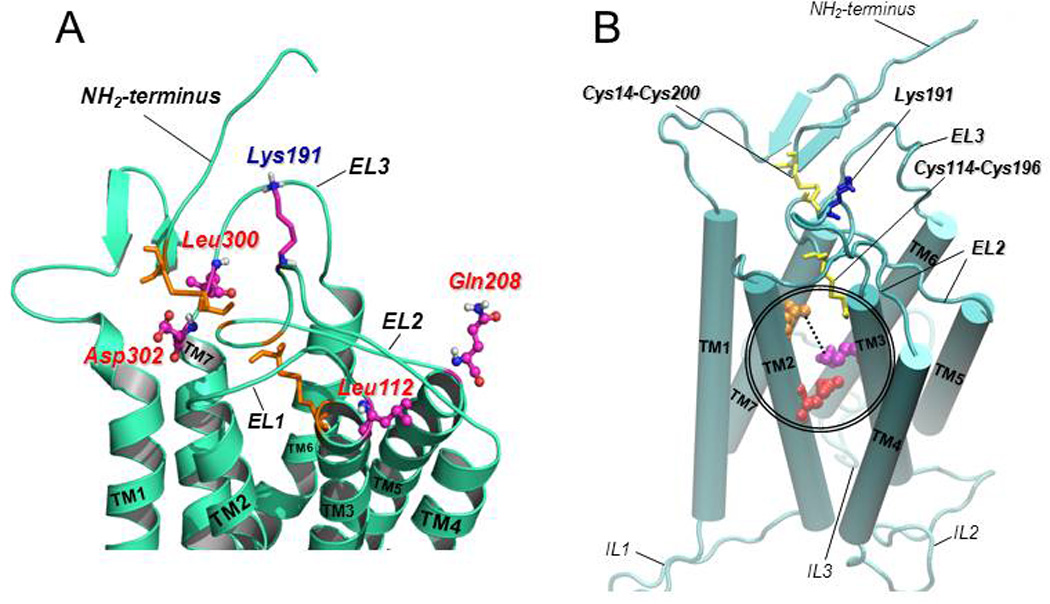
A: Predicted structure of the upper-third portion of the hGnRHR based on homology modeling with the structure of bovine rhodopsin (Jardon-Valadez et al., 2008). The coiled structures represent the antiparallel α-helices of transmembrane domains 1 to 7 connected by the extracellular loops (EL) of the receptor (curved cords). Disulfide bonds between Cys14 (at the NH2-terminus) and Cys200 (at the EL2), and between Cys114 (at the COOH-terminal end of the EL1) and Cys196 (at the EL2) are shown as orange sticks. The location of the amino acid residues that represent a motif of four non-contiguous residues at positions 112 (Leu, at the EL1), 208 (Gln, at the EL2), 300 (Leu, at the EL3), and 302 (Asp, at the EL3) that presumably control the destabilizing role of Lys191 (shown as purple, blue and grey sticks, at the EL2) on the association of the NH2-terminus and the EL2 and subsequent formation of the Cys14–Cys200 bridge are shown in colored circles and sticks. B: Predicted model of the hGnRHR showing the seven transmembrane helices (displayed as rods) connected by the extracellular (EL) and intracellular (IL) loops (Jardon-Valadez et al., 2008). Cys14–Cys200 and Cys114–Cys196 disulfide bridges are shown as yellow sticks; Lys 191 is represented by blue sticks. Glu90 (at the TM2; red spheres) forms a salt bridge with Lys121 (at the TM3; purple spheres) which is eliminated by the Glu90Lys mutation. Pharmacoperones act to stabilize the Glu90Lys mutant by bridging residues Asp98 (at the extracellular face of TM1; orange spheres) and Lys121 (discontinuous line).
