Abstract
Sensors that change color have the advantages of versatility, ease of use, high sensitivity, and low cost. The recent development of optically based chemical sensing platforms has increasingly employed substrates manufactured with advanced processing or fabrication techniques to provide precise control over shape and morphology of the sensor micro- and nano-structure. New sensors have resulted with improved capabilities for a number of sensing applications, including the detection of biomolecules and environmental monitoring. This perspective focuses on recent optical sensor devices that utilize nanostructured substrates.
Keywords: Colorimetric Sensors, Diffraction Gratings, Photonics, Plasmonics, Nanostructures, Sensors
Over the past decade, a variety of new optical sensing modalities have been developed, catalyzing research in both fundamental and applied sciences. Current research has increasingly exploited advances in the fabrication of materials to modify and greatly enhance existing sensing techniques. Greater control over the micro- and nano-structure of materials has resulted in sensor materials with increased sensitivities, multiplexing capabilities, or both. For example, while surface plasmon resonance has been known for 25 years, advances in lithographic techniques have enabled additional control over the fabrication of nanostructures and has opened up new paradigms in probing molecular interactions due to the heightened sensitivity these structures provide. In addition, advances in silicon microfabrication have given rise to a wide variety of new structured materials such as photonic resonators with high Q-factors. As another example, colorimetric indicators were a staple in analytical chemistry long before instrumental analysis became commonplace; new structured sensor materials now incorporate colorimetric dyes, enhancing their properties and expanding their applicability. In this review, we highlight a number of recent developments of structured substrates for optically-based sensing, particularly involving methods that detect analytes through differences in wavelength dependent material properties.
Surface Plasmon Sensing Substrates
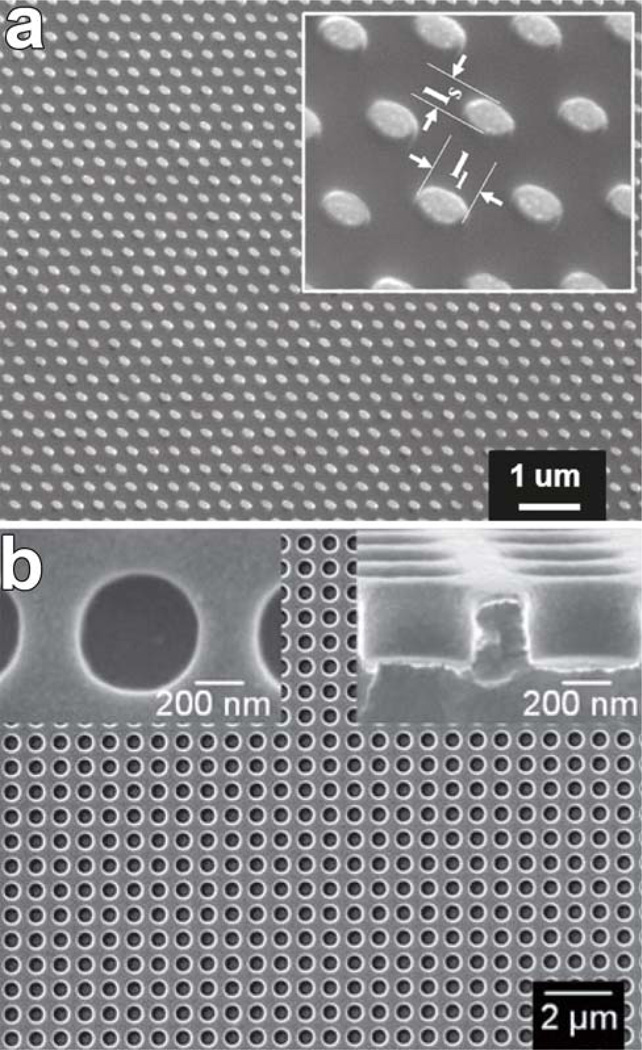
Conventional surface plasmon resonance relies on changes in the bulk refractive index of the surrounding medium. Localized surface plasmon resonance (LSPR), in contrast, probes the local dielectric environment immediately surrounding a plasmonic nanostructure.1–4 By optimizing the size and shape of nanostructures, the optical properties can be extremely sensitive to surface binding events that change the local refractive index. An assortment of structured metal substrates with well-controlled size and spacing have been employed for sensing with LSPR such as nanoholes, nanowells, and other nanocrystalline shapes; representative structures are shown in Figure 1.
Figure 1.
(a) Array of elliptical Au nanodisks on a glass wafer; inset shows higher magnification and the aspect ratio of the nanodisks (from ref. 8). (b) Scanning electron micrographs of a nanowell plasmonic crystal (from ref. 7). Left inset: Top view showing approximate nanowell diameter. Right inset: Cross-sectional view showing nanowell depth and continuous Au coverage on the surface.
A common sensing motif in LSPR involves functionalizing the surface of the nanostructure with an antibody or an antigen, and introducing a sample containing the target analyte. The specific binding of the analyte, as dictated by the selectivity of the capture agent, changes the local refractive index proximal to the nanostructure, which is then measured as a modulation in the wavelength-dependent optical properties of the nanostructure This works effectively for a variety of antibody-antigen pairs, including gold nanoislands functionalized with rabbit immunoglobulin G and human chorionic gonadotropin,5 nanohole arrays functionalized with glutathione s-transferase,6 and nanowell arrays functionalized with antigoat immunoglobulin G.7
Sensitivities of antibody-antigen pairs can be increased by using sandwich assays with modified detection antibodies. Detection antibodies for prostate-specific antigen (PSA), for example, were modified with alkaline phosphatase, which catalyzed the production of insoluble precipitates that agglomerated on an elliptical nanodisc substrate.8 While the PSA binding events would have been otherwise undetectable at low concentrations (below 2.8 nM) due to the low surface coverage of the antigen, the build-up of precipitate on the nanostructure itself caused a refractive index change that results in femtomolar detection limits of the antigen. While the addition of a secondary amplification step allows for a significant improvement in signal, this does remove the ability to monitor the binding of a target antigen in real-time.
Enhanced LSPR signals have also been achieved by using nanoparticle-conjugated detection antibodies, increasing anti-biotin signals by 400%.9 This phenomenon is due to local refractive index changes in combination with plasmonic coupling between the nanostructure and conjugated nanoparticle.
LSPR is capable of detecting more than antibody-antigen binding, however. Van Duyne and coworkers have developed triangular nanoprism sensors to monitor drug interaction with a cytochrome p450 enzyme,10 to observe the conformational changes of a bound protein,11 and even to directly detect adsorption of inert gases (i.e., He, Ar, N2) on non-functionalized surfaces.12 Larson et al. have also displayed multiplexed detection of bovine serum albumin, NaCl, Coomassie blue, and liposome solutions with nanohole arrays.13 Similarly, Rogers, Nuzzo and coworkers have demonstrated that non-functionalized nanowell and nanopost plasmonic crystals show optical sensitivities to different alkanethiols14 as well as non-specific binding of proteins.7,15
Diffraction Grating Substrates
Diffraction gratings are another class of optical sensors that have benefited from advanced fabrication techniques. By monitoring the change in intensity of the diffraction spots from a grating, researchers can probe interactions occurring on the surface of or within the grating material.16,17 This class of sensors offers a number of advantages, including an extraordinarily simple read-out, ease of fabrication, and low cost. The gratings can also be fabricated using a variety of materials, enabling the use of a wide range of functionalization schemes.
Wark et al.18 developed a method of optical sensing based on nanoparticle enhanced diffraction gratings that takes advantage of the coupling of plasmons between a gold diffraction grating and gold nanoparticles. In this method, diffraction gratings (consisting of 7 µm wide and 45 nm thick gold lines) were lithographically defined on a glass substrate. The gratings were functionalized to present a single-stranded DNA (ssDNA) capture probe that was complementary to half of a target ssDNA. The target ssDNA was presented in solution to the sensor surface, where it bound to the capture probe. This binding event itself was not sufficient to elicit a response from the diffraction gratings. Afterwards, the surface was exposed to a solution of gold nanoparticles functionalized with a second ssDNA molecule that was complementary to the other half of the target ssDNA; upon binding to the target, the gold nanoparticles with the second ssDNA formed a sandwich pair bound to the functionalized grating. The coupling of localized surface plasmons on the gold diffraction grating surface to those of the nanoparticles resulted in a highly sensitive sensor. The gratings were used as a sensing substrate by examining the change in first order diffraction efficiency that occurs upon binding of target molecules to the surface of the gratings.18 This technique was further developed to incorporate enzymatic amplification steps that could lead to the incorporation of nanoparticles to the sensor surface.19
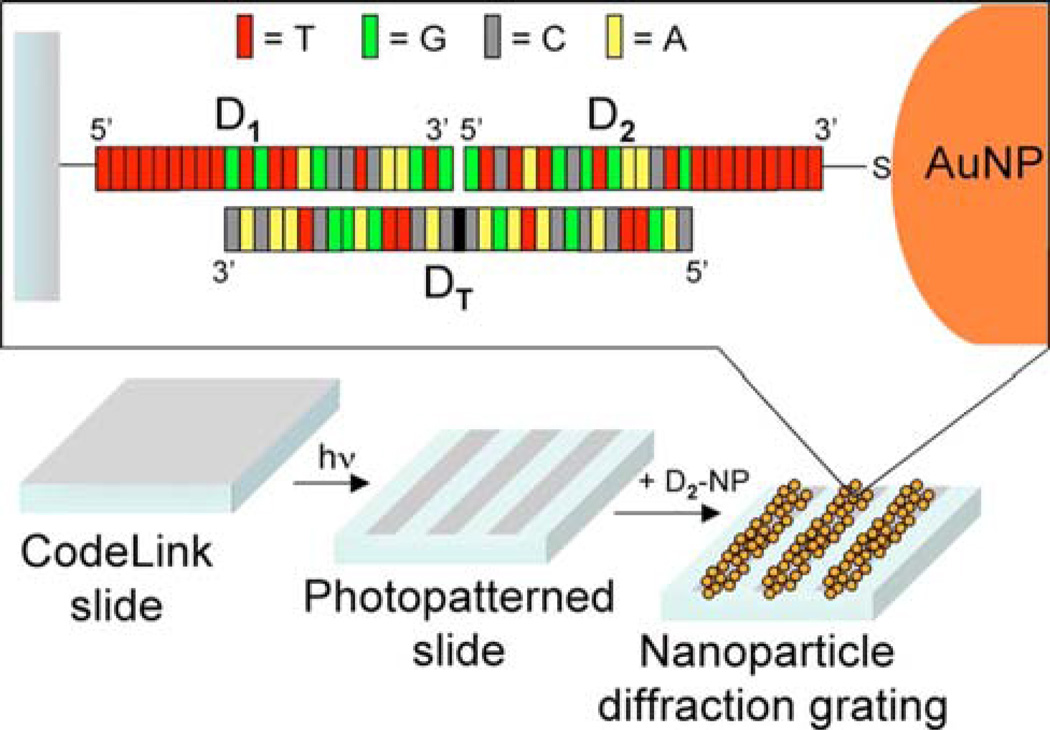
Another sensing method using diffraction gratings (shown in Figure 2) was conceived by Sendroiu and Corn.20 Instead of starting with a patterned metallic diffraction grating directly on the sensor substrate, the grating was assembled in situ onto 7.5 µm lines of ssDNA capture probes (sequence D1) patterned onto the surface of a glass slide. Hybridization of the target sequence (sequence DT) alone was not sufficient to elicit a diffraction response, but when the target was introduced after pre-associating with gold nanoparticles presenting a sequence partially complementary to the target (sequence D2), the resulting sandwich complex gave a diffraction response. By monitoring the first-order diffraction measurements with simple collimated white light, Sendroiu and Corn were able to detect target DNA at concentrations as low as 10 pM. By using a HeNe laser and avalanche photodiode, they were able to minimize background light scattering and reduce their limit of detection to 10 fM.
Figure 2.
Schematic diagram of the in situ generated nanoparticle diffraction gratings used for detecting ssDNA. In this method, ssDNA capture probes (D1) are patterned into lines on a glass substrate. The addition of the ssDNA target (DT) does not create a measurable signal by itself. Instead, addition of gold nanoparticles modified with the ssDNA probe sequence (D2) results in the formation of a diffraction grating, which can be read out optically (from ref. 20).
Photonic Crystals
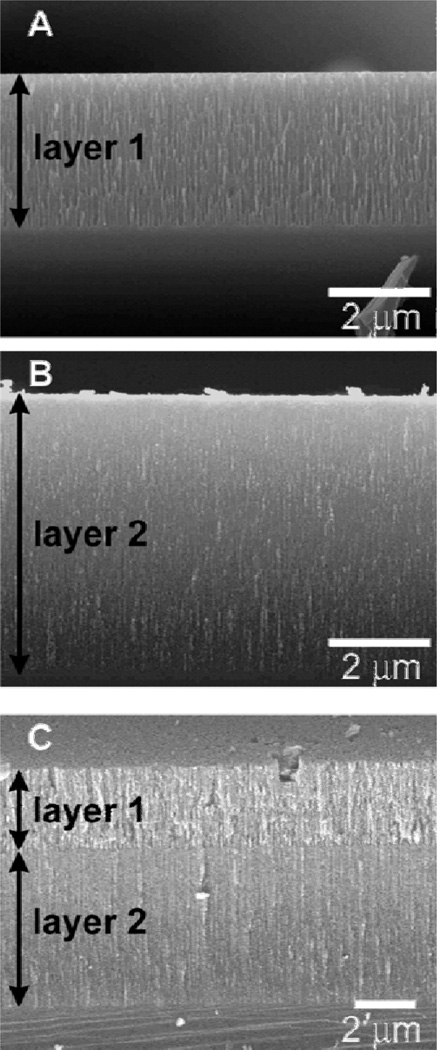
Photonic crystals are materials with periodic nanostructure regions of high and low dielectric constants that can attenuate light propagation due to conditions of constructive and destructive interference. This phenomenon can be accurately explained by Bragg’s law, mλ = 2nd sin(θ), where m is the diffraction order, λ is the wavelength of light, d is the lattice spacing of the material, n is the refractive index of the material, and θ is the incident angle of light. 1D photonic crystals, also called Bragg stacks or Bragg mirrors, are popular sensing materials that change color according to refractive index changes caused by adsorption or biding events in the material.21–24 Sailor and coworkers have developed 1D porous silicon photonic crystals by the electrochemical etching of crystalline silicon with hydrofluoric acid. By alternating the current during the etching process, pore densities can be controlled as a function of depth into the silicon (Figure 3).25 This precise control allows for a range of porous silicon materials to be synthesized, from those containing simple cylindrical pores to materials with oscillating, periodic pores. Materials containing these periodic structures are classified as a special type of 1D photonic crystal known as a Rugate filter. Rugate filter materials have been tailored by treating the surface of the pore walls with different functional groups and multiplexed for the detection of isopropanol and heptane vapors.26 A similar type of surface functionalization was used for the detection of HF and Cl2.27
Figure 3.
Scanning electron micrographs (secondary electron) of cross-sections of porous silicon single layers with (a) large and (b) small pore diameters. (c) By varying the applied current density during the etching process, double-layers can be also generated (from ref 25).
Double stacked porous silicon materials have been used by the Sailor group to detect ethanol, heptane, toluene, and dimethyl methylphosphonate (a simulant for sarin gas).28 A similar double layered silicon material has also been used to monitor the enzymatic activity of Pepsin through the digestion of α-casein.29 Encoded multilayered porous silicon surfaces were also used for the multiplexed detection of DNA (Figure 4).30 Since different etching currents result in characteristic reflectance properties, a combination of characteristic peaks can be encoded into a material by applying a specific current sequence to the etch process, creating a “spectral barcode” within the material. Particular DNA capture sequences can be covalently attached to a silicon substrate with a specific spectral-barcode. A mixture of target DNA is fluorescently labeled, and subsequently allowed to hybridize with the silicon substrates. Monitoring the fluorescence can detect when a binding event occurs, and reflectivity measurements can determine which specific DNA sequence is fluorescing. This allows for the detection and discrimination of multiple analytes within the same sample. Lee et al. have also used multi-layered porous silicon photonic crystals for the detection of a series of polar solvents, such as isopropanol, ethanol, methanol, and acetone (Figure 5).31,32
Figure 4.
Scanning electron micrograph of porous silicon substrates used for multiplexed detection of DNA (from ref. 30).
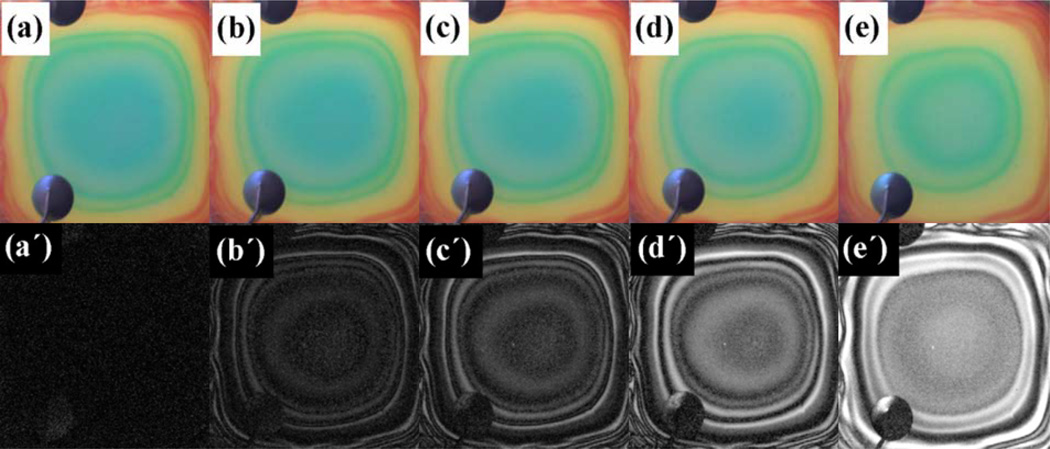
Figure 5.
Optical photographs (a through e) and difference images (a´ through e´) of a porous silicon Bragg mirror exposed to ethanol vapor at concentrations of (a) 0 ppm, (b) 140 ppm, (c) 2000 ppm, (d) 10,000 ppm and (e) 22,500 ppm. The difference images are multiplied by a factor of 10 to show the changed segments of color clearly (from ref. 31).
Hybrid photonic crystals have also been produced for optical detection of a variety of analytes. Míguez and coworkers have developed Bragg reflector materials based on alternating SiO2-TiO2 layers for the detection of isopropanol, water, toluene, and chlorobenzene.33,34 De Stefano et al. used a porous silicon photonic crystal with amino-functionalized poly(ε-caprolactone) for the detection of isopropanol, ethanol and methanol.35 The polymer coating was found to protect the silicon from alkaline dissolution without adversely affecting detection limits. The Sailor group used a porous silica photonic crystal backfilled with an acrylic hydrogel for the optical detection of pH and temperature changes.36
Ozin and coworkers have developed an alternative bottom-up synthetic pathway to 1D photonic crystals using nanoparticle assemblies (Figure 6). Alternating films of mesoporous silica and titania nanoparticle films were capable of optical detection of varying pressures of toluene vapor.37 Alternating α-Fe2O3/WO3 nanoparticle films and ZnO/WO3 nanoparticle films were used in a similar fashion for the optical discrimination of ethanol, isopropanol, n-propanol, and t-butanol.38 Additionally, alternating SiO2 and TiO2 nanoparticle layers with nine different surface functionalities have been used for the multiplexed detection and discrimination of six different volatile organic solvents and four different bacteria strains.39
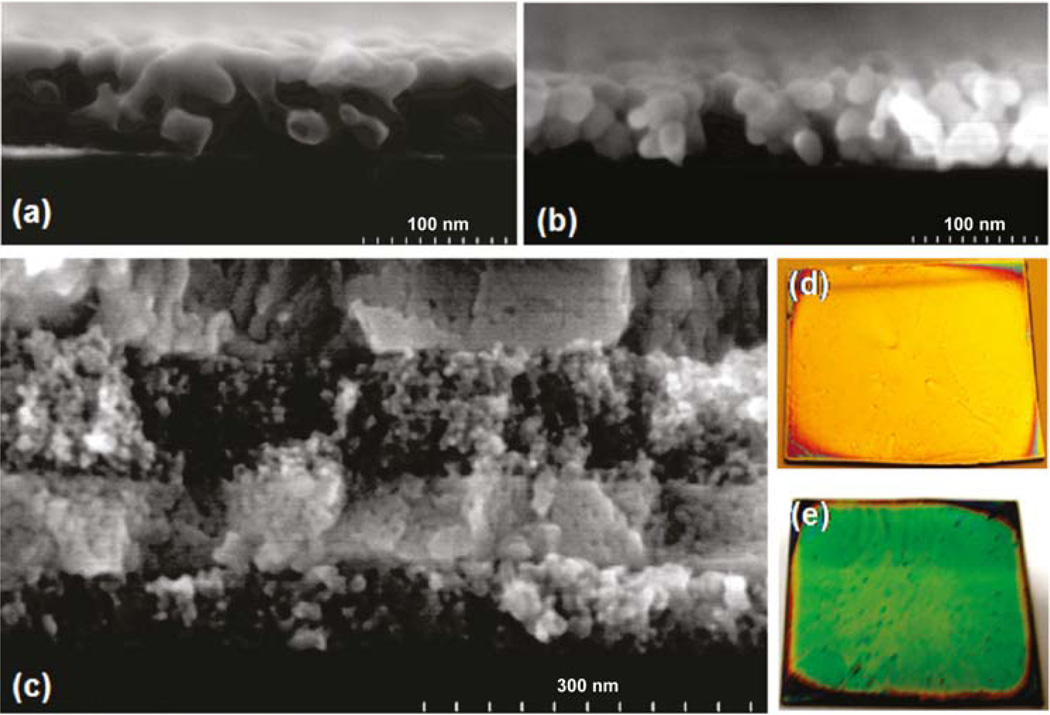
Figure 6.
(a) SEM cross section image of a porous nanoparticle α-Fe2O3 thin film; (b) SEM cross-section image of a porous nanoparticle ZnO thin film; (c) SEM cross-section image of two double layers of a nanoparticle α-Fe2O3/WO3 Bragg mirror; (d) optical photograph showing yellow reflectivity of spin-coated three double layer ZnO/WO3 Bragg mirror; (e) optical photograph showing green reflectivity of spin coated three double layer nanoparticle α-Fe2O3/WO3 Bragg mirror (from ref. 38).
Photonic Resonators
Another class of rationally structured sensors that take advantage of microscale lithography are photonic resonators.40,41 Light is coupled into a dielectric material via the evanescent field of a neighboring optical fiber or waveguide. Light at specific wavelengths can couple into the microcavity at specific wavelengths, which are defined by a constructive interference condition: mλ = 2πrneff where m is a non-zero integer, λ is the wavelength of incident light, r is the radius of the resonator, and neff is the effective refractive index of the optical mode. The refractive index term of the resonance condition renders these devices sensitive to binding induced changes in the local dielectric environment, often in the form of target biomolecules binding to specific receptors (DNA, antibodies, etc.).
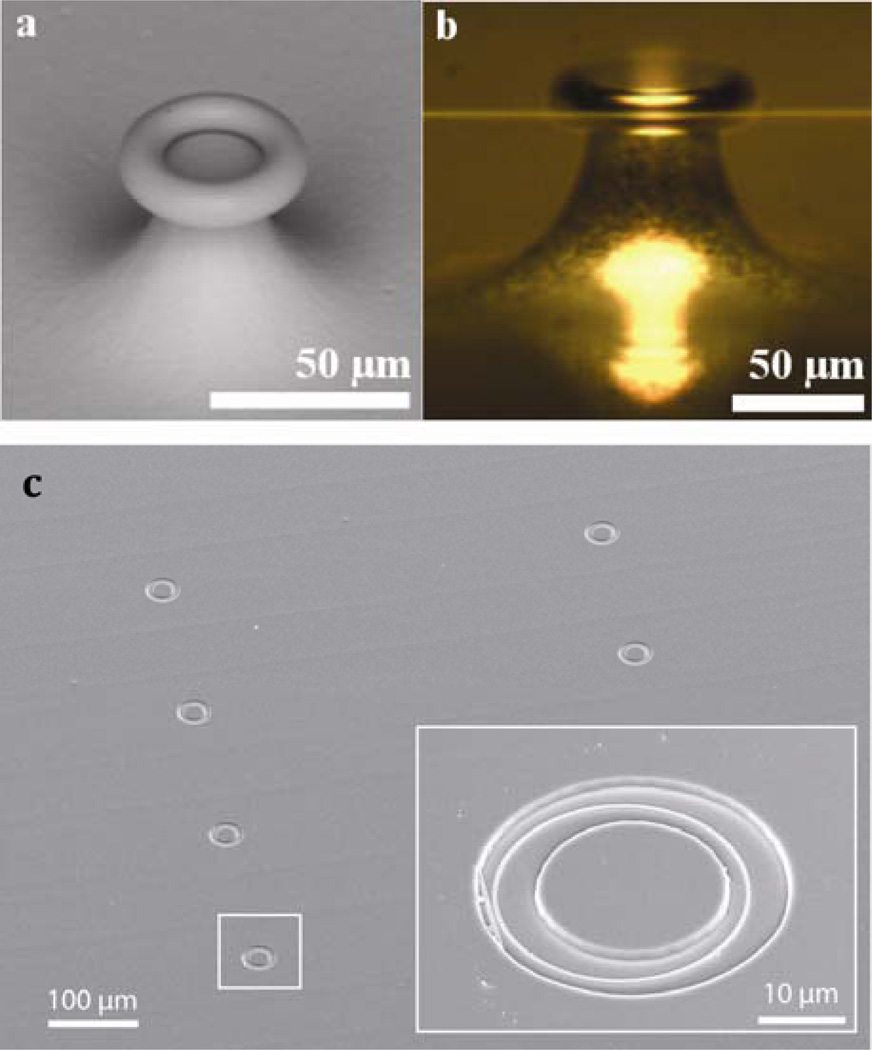
These sensors have been realized via microfabrication in several of different geometric configurations. Notably, high fidelity fabrication methods can allow for exceptionally high quality factor cavities, and thus small shifts in the exceptionally spectrally narrow resonances can be easily resolved. Microtoroids (Figure 7a) can offer exceptionally high quality factors and the possibility of single molecule detection has been suggested using these devices.42,43 Optical interrogation of these devices, however, remains a challenge at present, and this limits their applicability in multiplexed sensor configurations.
Figure 7.
(a) Scanning electron micrograph and (b) an optical micrograph of a microtoroid photonic resonator (from ref 43). A tapered fiber used to excite resonance in the microtoroid can be seen in (b) as well. (c) An array of microring resonators from ref. 47. The inset shows an individual microring next to a linear waveguide, both which have been revealed within an annular opening inside a polymer cladding layer.
An alternative photonic resonator is based on chip-integrated microring waveguides (Figure 7b). These structures typically feature lower quality factors (Q≈105), but offer key advantages in terms of fabrication and optical interrogation.44,45 In fact, large arrays of individually addressable microrings can be easily fabricated using standard commercial semiconductor processing methods, and the lithographically controlled positioning of the linear access waveguide next to the microring greatly simplifies optical alignment. Utilizing microring resonators, Bailey and coworkers have demonstrated a wide range of sensing array applications, including the multiplexed detection of proteins,46 nucleic acids,47 and cytokines.48 Silicon photonic wires for protein and nucleic acid detection have also been fabricated in spiral geometries that offer increased path length while maintaining a compact footprint.49–51
Colorimetric Sensor Substrates
Structured substrates are also used in colorimetric detection methods (i.e., those involving chemically responsive dyes or pigments) and can be easily adapted to a variety of analytes. As discussed below, colorimetric detection methods have the advantage of being exceptionally inexpensive due to the ubiquitous nature of digital photographic imaging, which also allows them to be highly portable. Due to their rapid recent development, as well as their versatility, portability, and low-cost, the remainder of this review will focus on colorimetric sensors. There has also been substantial recent colorimetric research using surface functionalized nanoparticles where optical properties change due to the distance-dependent interactions of nanoparticle aggregates; these materials will not be reviewed here, having been thoroughly discussed elsewhere by Mirkin,52 Lu,53 and Rotello.54
A central requirement of all colorimetric sensors is analyte access to the chromophores. This can be met with semi-liquid films of highly plasticized polymers containing dissolved indicators, but it is often better served by the use of high porosity membranes as nanostructured supports. Porosity on the nanometer scale is critical for rapid exposure of colorimetric indicators to the environment containing potential analytes.55 One may even argue that litmus paper is the predecessor to all sensors with structured substrates, given the microfibrous nature of cellulose paper. More recently, fibrous membranes made from other polymers have found important applications for colorimetric sensors. PVDF (polyvinylidene difluoride, i.e., –(CH2CF2)n–) has found to be particularly effective for a variety of biochemical applications (e.g., western blots) due to its high purity, chemical inertness and ease of fabrication. Membranes of PVDF are made by floating a solution of the polymer dissolved in dimethylformamide or methyl ethyl ketone on a water trough; the fibrous membrane is formed on the water surface as the organic solvent diffuses into the water.
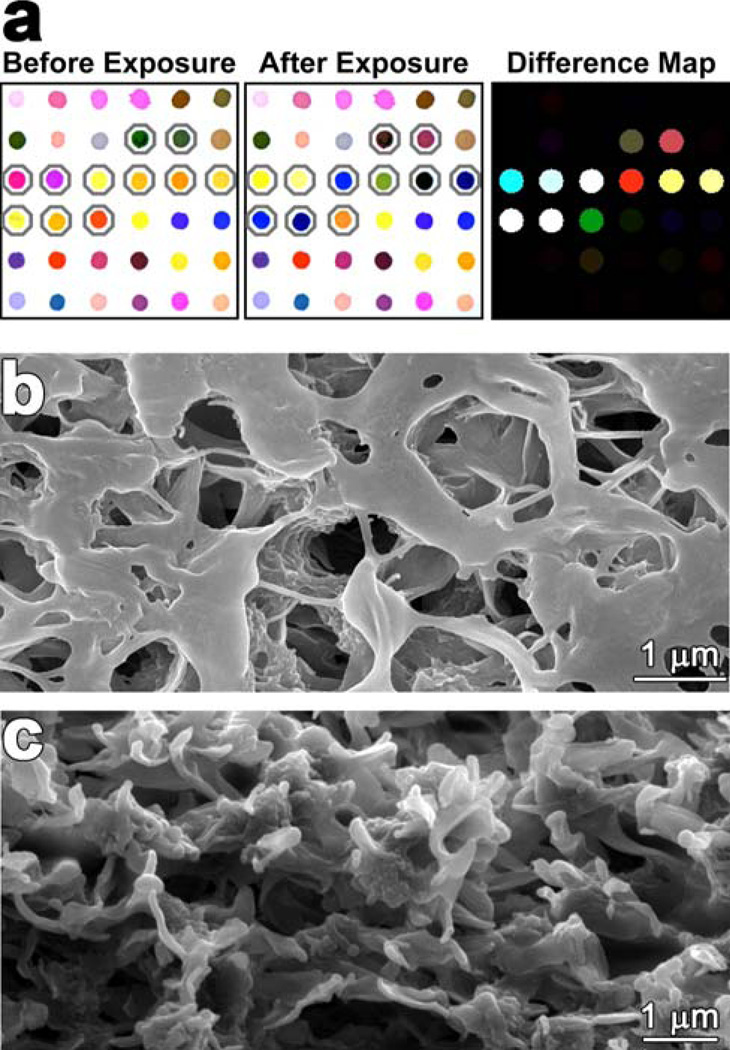
Over the past few years, a general approach to an “optoelectronic nose” developed that used a diverse range of chemically responsive dyes deposited on high surface area PVDF membranes.55–64 This approach uses disposable colorimetric sensor array with selected chemically-responsive dyes in four classes: (1) dyes containing metal ions (e.g., metalloporphyrins) that respond to Lewis basicity (i.e., electron-pair donation, metal-ion ligation), (2) pH indicators that respond to Brønsted acidity/basicity (i.e., proton acidity and hydrogen bonding), (3) dyes with large permanent dipoles (e.g., vapochromic or solvatochromic dyes) that respond to local polarity, and (4) metal salts that participate in redox reactions. This colorimetric sensor array, therefore, is responsive to the chemical reactivity of analytes, rather than to their effects on the secondary physical properties (e.g., mass, conductivity, adsorption, etc.) normally used by other electronic nose techniques. The colorimetric sensor array is simply digitally imaged before and after exposure, and a difference map (red minus red, green minus green, blue minus blue) is generated (Figure 8a). As discussed below, the difference map is a unique molecular fingerprint for each odorant or mixture of odorants at a given concentration.
Figure 8.
(a) Colorimetric sensor array printed on PVDF membrane, showing the digital images before and after exposure to an analyte (in this case, ammonia at its IDLH concentration) and the color difference map; (b) SEM image of the PVDF top surface after printing; (c) SEM image of the cross-section of the printed PVDF membrane. Modified from ref. 65.
The indicator formulations did not clog the pores or damage the PVDF morphology upon printing, as shown by scanning electron micrographs of printed (Figure 8bc) and non-printed areas of the membrane.65 Printing of the arrays on PVDF did not alter the porosity of the membrane in any way or damage or dissolve the PVDF membrane, and the microstructure of the membrane was completely unaffected.
In general, ordinary pigments (i.e., insoluble colorants) cannot be used as sensors for the simple reason that analytes can interact only with the very external monolayer of the colorant. If the pigment is created so that it has a very high microporosity, however, then analytes can gain access to the internal colorant centers. Such nanoporous pigments have generally been synthesized using various colorimetric indicators doped into organically modified silicas (ormosils) for an assortment of sensing applications.66–68 These materials circumvent complex processing steps by simply allowing an amorphous silica matrix to form around the indicator molecules, effectively trapping them within the solid as condensation occurs, while allowing access of the chromophore to environmental analytes.
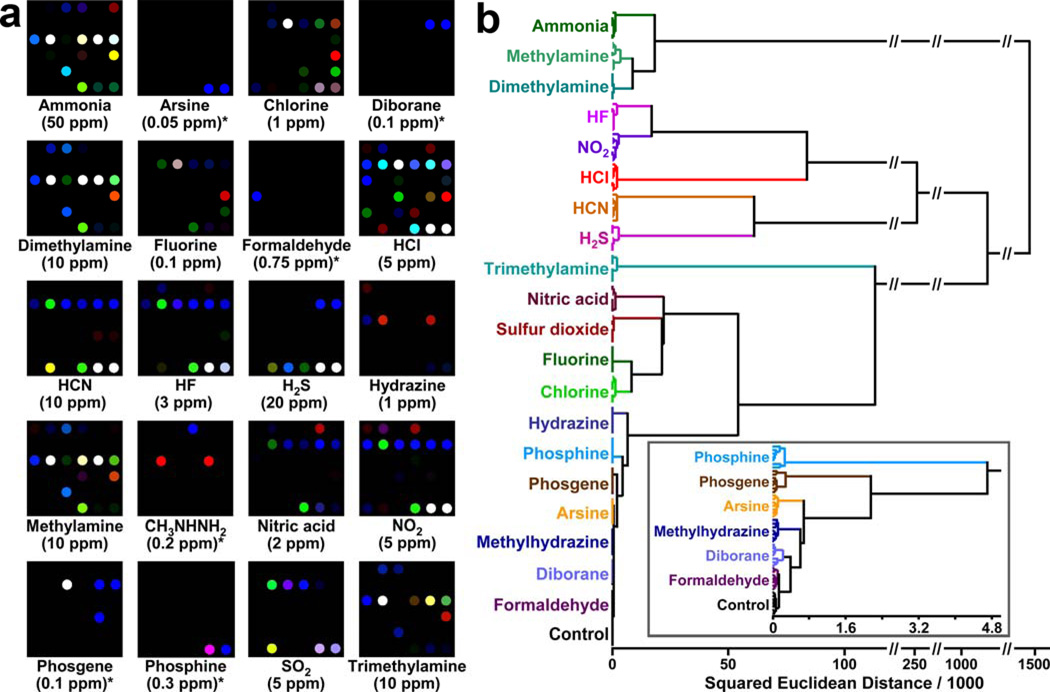
The Suslick group has used these sol-gel materials to develop printable colorimetric sensor arrays based on chemically-responsive nanoporous pigments.65 By using inks made from ormosil suspensions containing chemically responsive dyes, they are able to print arrays onto nearly any nonporous surface. By combining differently doped porous pigments into one array, the composite response of the immobilized semi-specific indicators to environmental changes can be used to provide a “molecular fingerprint” unique to the analyte, in much the same way that the olfactory system works. The molecular fingerprint is used to visually differentiate similar analytes, and the high dimensionality allows for the facile discrimination among diverse analytes (Figure 9).
Figure 9.
Nanoporous ormosil based colorimetric sensor arrays. (a) Color difference maps and (b) hierarchical cluster analysis of twenty representative toxic industrial chemicals (TICs) at their permissible exposure level (PEL) concentration. For display purposes, the color range of these difference maps are expanded from 4 to 8 bits per color (RGB range of 4–19 expanded to 0–255), except for several weaker responding TICs that are marked with asterisks (RGB range of 2–3 expanded to 0–255) (from ref. 72).
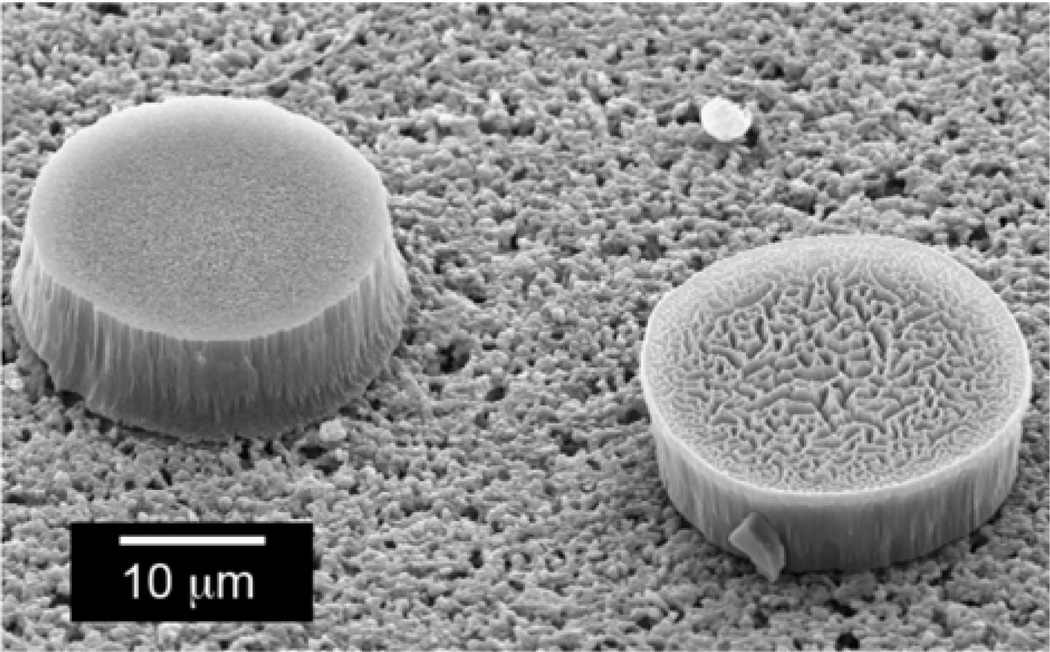
The nanoporous ormosil materials are prepared by acid-catalyzed hydrolysis of solutions containing commercially available silane precursors (e.g., tetraethoxysilane (TEOS), methyltriethoxysilane (MTEOS), phenethyltrimethoxysilane, and octyltriethoxysilane (octyl-TEOS)) and dissolved in low volatility solvents (e.g., methoxyethanol or diethylene glycol dimethyl ether) that serve as porogens on the nanometer scale. After hydrolysis, the chemically responsive indicators are added to the sol–gel solutions, printed on a polymer or glass surface, and allowed to form a porous xerogel.69 After curing, the resulting porous pigment is ~1 mm in diameter and ~4 µm thick, and consists of 50–200 nm pores throughout the material (Figure 10). Arrays of these porous pigments have been used for the detection of toxic industrial gases at ppb concentrations,70–72 complex odorant mixtures (e.g., coffee aromas),73 and sugars and artificial sweeteners in aqueous solutions.74,75
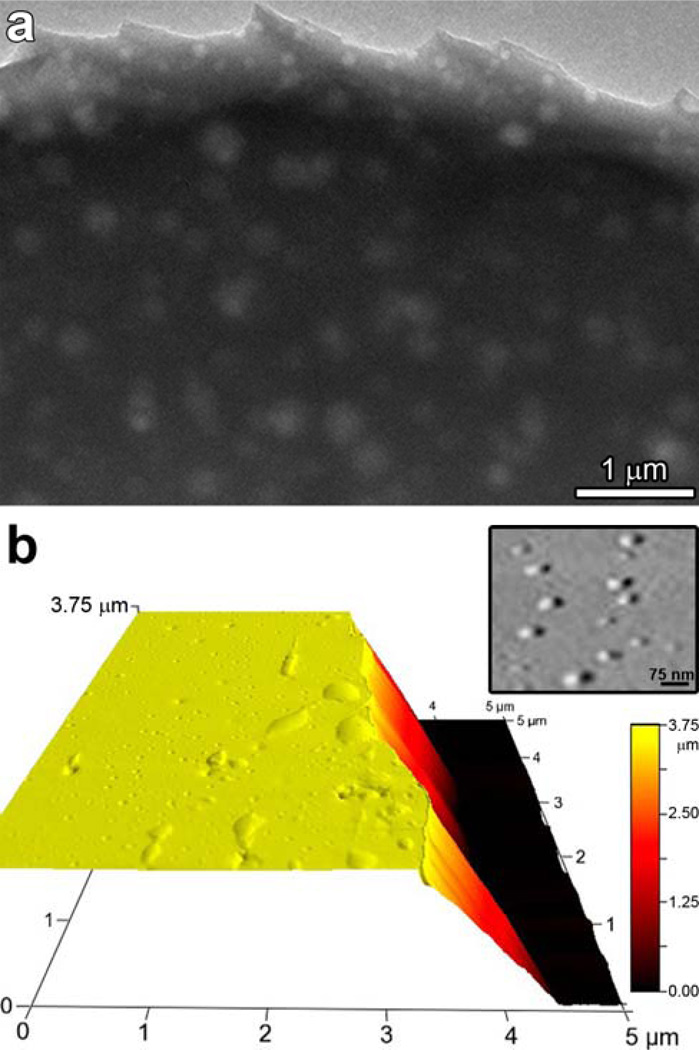
Figure 10.
(a) Transmission electron micrograph of printed porous pigment which was then removed from the polymer substrate and placed on a TEM grid. The 50 to 200 nm features show the porosity created in these ormosil xerogel spots, which are ~4 µm thick and 1 mm in diameter. (b) An AFM micrograph in perspective showing the height of the porous pigment at the spot center compared to the base height of the PET as revealed by a scrape from a blade. Inset shows an enlargement of the surface pores (< 100 nm) (from ref. 69).
As noted earlier, high porosity on the nanometer scale is critical for rapid exposure of colorimetric indicators to the environment containing potential analytes. To this end, the development of periodic mesoporous silica materials has also proved of use for some colorimetric sensors. Silica has a number of advantages as a host material for colorimetric probes including optical transparency, relative inertness to both gasses and liquids, good stability over a wide range of pH, and high surface areas. Highly ordered mesoporous aluminosilica materials are typically synthesized using structure-directing surfactant templates, such as CTAB (cetyltrimethylammonium bromide) or triblock copolymers. These sacrificial templates form columnar micelles which direct silica condensation, resulting in the formation of highly periodic and highly tunable porous materials.76,77,78 There are also a few downsides to using highly ordered mesoporous materials for colorimetric sensing applications: the sacrificial templates are relatively expensive and the synthesis complicated by the washing or calcination needed for template removal, which complicates colorimetric indicator incorporation and generally precludes easy preparation of sensor arrays.
After template removal, the resulting mesostructured silicas can be functionalized or doped with reactive chromophores for colorimetric sensing and such materials have recently been used for metal ion sensing especially, but also for a range of liquid and gas phase organics. Jung and coworkers have developed mesoporous silica based colorimetric sensors for the detection of Cu2+ and Hg2+ cations based on covalently tethered chromophores.79,80 Similarly, El Safty et al. have demonstrated Co2+, Cu2+ and Hg2+ detection using electrostatically immobilized indicators.81–83 Martínez-Máñez and coworkers have recently reported five different mesoporous silica based sensors used in a multiplexed system for the detection and discrimination of 12 biologically relevant anions (e.g., glutamate, ADP, ATP) at physiological pH.84 This group has also recently produced Hg2+ and pyrophosphate sensors using immobilized squarine dyes85,86 in addition to a size-selective amine sensor.87 Johnson and coworkers have used phenyl containing silica materials further functionalized with porphyrin indicators for the detection of hydrocarbons such as benzene, toluene, cyclohexane and hexane.88,89 Li and Stein developed a hierarchical material where mesoporous silica spheres containing immobilized tetra(1-methyl-4-pyridal)porphyrin were entrapped in a periodic mesoporous silica skeleton that was capable of detecting Cd2+ ions at low ppb concentrations (Figure 11).90 This two-stage approach could allow for the embedding of mesoporous spheres containing functionalities that may not otherwise be compatible with the host silica material.
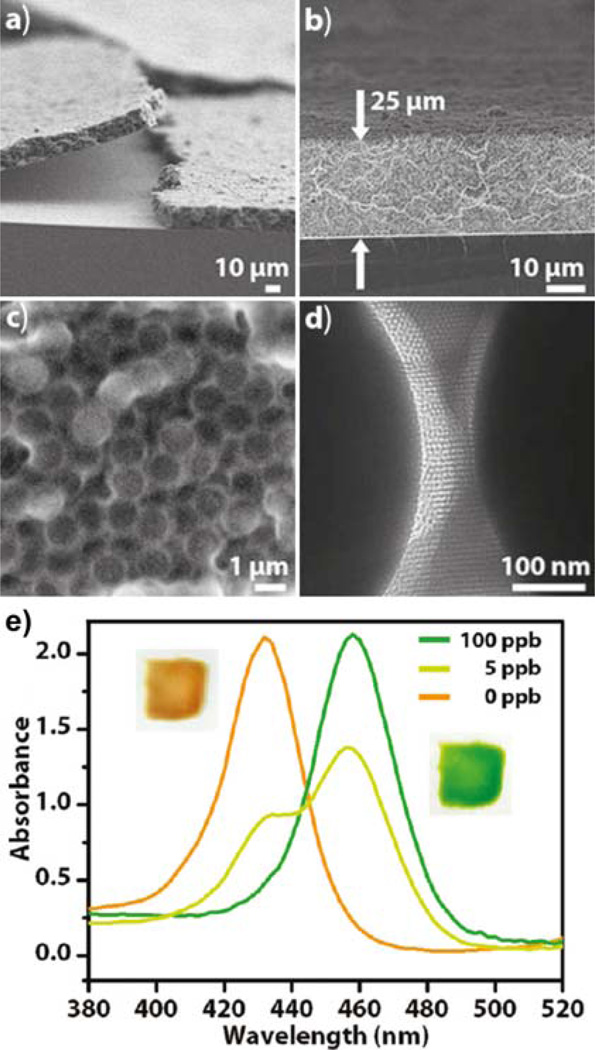
Figure 11.
(a) SEM image showing an overview of the composite film of mesoporous silica spheres in a mesoporous silica framework (from ref. 90). (b) Cross-sectional SEM of the composite film. (c) SEM showing the composite structure of spheres within the silica matrix. (d) TEM revealing the mesoporous structure in the silica matrix used to embed the spheres. (e) UV-vis absorption spectra of the composite exposed to solutions containing the indicated concentrations of Cd2+ ions. The insets show digital photographs of the sensing materials as synthesized (left) and in a 20 ppb Cd2+ solution (right).
Conclusion
An assortment of optically-based chemical sensing techniques have benefited from the use of micro- and nano- structured substrates, resulting in devices with improved detection performance. Most such sensors have been developed for the detection of a single analyte, particularly in bioassays utilizing antibody-antigen pairs, while multiplexed systems are generally in an earlier stage of implementation. Substrate specificity remains a challenge, particularly for low level detection of analytes in the complex milieu that biofluids generally contain. The alternative method of chemical specificity from pattern recognition of sensor arrays has proved especially useful in environmental analysis in both gas and liquid phase analysis.
Quotes to Highlight in Paper.
“Current research has increasingly exploited advances in the fabrication of materials to modify and greatly enhance existing sensing techniques. Greater control over the micro- and nano-structure of materials has resulted in sensor materials with increased sensitivities, multiplexing capabilities, or both.”
“In this review, we highlight a number of recent developments of structured substrates for optically-based sensing, particularly involving methods that detect analytes through differences in wavelength dependent material properties.”
“An assortment of optically-based chemical sensing techniques have benefited from the use of microand nano- structured substrates, resulting in devices with improved detection performance.”
Acknowledgments
This work was supported in part by NIH (ES016011) and by NSF (DMR 0906904).
Biographies
Jonathan Kemling obtained his B.S. in Chemistry from Michigan Tech in 2005, followed by his Ph.D. at the University of Illinois at Urbana-Champaign in 2011 working under Prof. Kenneth S. Suslick developing colorimetric sensors for numerous sensing applications. He is currently a Senior Analytical Chemist for 3M.
Abraham Qavi is a graduate student at the University of Illinois at Urbana-Champaign. He obtained a B.S in Biochemistry & Molecular Biology and a B.S. in Chemistry from the University of California, Irvine. He is completing a M.D. and a Ph.D. in Chemistry under the supervision of Prof. Ryan Bailey, where he is utilizing arrays of silicon photonic resonators for nucleic acid detection.
Ryan Bailey (http://www.scs.illinois.edu/bailey/Home.html) is an Assistant Professor of Chemistry at the University of Illinois at Urbana-Champaign. He completed his B.S. in Chemistry from Eastern Illinois University, and obtained his Ph.D. from Northwestern University. He subsequently pursued a joint post-doctoral fellowship at the Institute for Systems Biology and the California Institute of Technology, after which he came to the University of Illinois. His research interests include the development of new sensing methodologies based on silicon photonics as well as the development of techniques for generating surface immobilized protein gradients to study a wide variety of cellular behavior.
Kenneth Suslick (http://www.scs.illinois.edu/suslick/) is the Marvin T. Schmidt Professor of Chemistry, Professor of Materials Science and Engineering, and Professor at the Beckman Institute for Advanced Science and Technology at the University of Illinois at Urbana-Champaign. He received a B.S. from the California Institute of Technology and Ph.D. from Stanford University, after which he joined the faculty at the University of Illinois. His research interests encompass a broad range of topics, including the chemical effects of ultrasound, colorimetric sensor arrays, and the bioinorganic chemistry of metalloporphyrins.
References
- 1.Englebienne P. Use of Colloidal Gold Surface Plasmon Resonance Peak Shift to Infer Affinity Constants from the Interactions between Protein Antigens and Antibodies Specific for Single or Multiple Epitopes. Analyst. 1998;123:1599–1603. doi: 10.1039/a804010i. [DOI] [PubMed] [Google Scholar]
- 2.Malinsky MD, Kelly KL, Schatz GC, Van Duyne RP. Chain Length Dependence and Sensing Capabilities of the Localized Surface Plasmon Resonance of Silver Nanoparticles Chemically Modified with Alkanethiol Self-Asembled Monolayers. J. Am. Chem. Soc. 2001;123:1471–1482. [Google Scholar]
- 3.Haes AJ, Van Duyne RP. A Nanoscale Optical Biosensor: Sensitivity and Selectivity of an Approach Based on the Localized Surface Plasmon Resonance Spectroscopy of Triangular Silver Nanoparticles. J. Am. Chem. Soc. 2002;124:10596–10604. doi: 10.1021/ja020393x. [DOI] [PubMed] [Google Scholar]
- 4.Stewart ME, Anderton CR, Thompson LB, Maria J, Gray SK, Rogers JA, Nuzzo RG. Nanostructured Plasmonic Sensors. Chem. Rev. (Washington, DC, U. S.) 2008;108:494–521. doi: 10.1021/cr068126n. [DOI] [PubMed] [Google Scholar]
- 5.Bendikov TA, Rabinkov A, Karakouz T, Vaskevich A, Rubinstein I. Biological Sensing and Interface Design in Gold Island Film Based Localized Plasmon Transducers. Anal. Chem. (Washington, DC, U. S.) 2008;80:7487–7498. doi: 10.1021/ac8013466. [DOI] [PubMed] [Google Scholar]
- 6.Yang JC, Ji J, Hogle JM, Larson DN. Metallic Nanohole Arrays on Fluoropolymer Substrates as Small Label-Free Real-Time Bioprobes. Nano Lett. 2008;8:2718–2724. doi: 10.1021/nl801043t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart ME, Yao J, Maria J, Gray SK, Rogers JA, Nuzzo RG. Multispectral Thin Film Biosensing and Quantitative Imaging Using 3D Plasmonic Crystals. Anal. Chem. (Washington, DC, U. S.) 2009;81:5980–5989. doi: 10.1021/ac900819j. [DOI] [PubMed] [Google Scholar]
- 8.Lee SW, Lee KS, Ahn J, Lee JJ, Kim MG, Shin YB. Highly Sensitive Biosensing Using Arrays of Plasmonic Au Nanodisks Realized by Nanoimprint Lithography. ACS Nano. 2011;5:897–904. doi: 10.1021/nn102041m. [DOI] [PubMed] [Google Scholar]
- 9.Hall WP, Ngatia SN, Van Duyne RP. LSPR Biosensor Signal Enhancement Using Nanoparticle-Antibody Conjugates. J. Phys. Chem. C. 2011;115:1410–1414. doi: 10.1021/jp106912p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das A, Zhao J, Schatz GC, Sligar SG, Van Duyne RP. Screening of Type I and II Drug Binding to Human Cytochrome P450-3A4 in Nanodiscs by Localized Surface Plasmon Resonance Spectroscopy. Anal. Chem. (Washington, DC, U. S.) 2009;81:3754–3759. doi: 10.1021/ac802612z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall WP, Modica J, Anker J, Lin Y, Mrksich M, Van Duyne RP. A Conformation- and Ion-Sensitive Plasmonic Biosensor. Nano Lett. 2011;11:1098–1105. doi: 10.1021/nl103994w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bingham JM, Anker JN, Kreno LE, Van Duyne RP. Gas Sensing with High-Resolution Localized Surface Plasmon Resonance Spectroscopy. J. Am. Chem. Soc. 2010;132:17358–17359. doi: 10.1021/ja1074272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang JC, Ji J, Hogle JM, Larson DN. Multiplexed Plasmonic Sensing Based on Small-Dimension Nanohole Arrays and Intensity Interrogation. Biosens. Bioelectron. 2009;24:2334–2338. doi: 10.1016/j.bios.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao J, Stewart ME, Maria J, Lee TW, Gray SK, Rogers JA, Nuzzo RG. Seeing Molecules by Eye: Surface Plasmon Resonance Imaging at Visible Wavelengths with High Spatial Resolution and Submonolayer Sensitivity. Angew. Chem. Int. Ed. 2008;47:5013–5017. doi: 10.1002/anie.200800501. [DOI] [PubMed] [Google Scholar]
- 15.Truong TT, Maria J, Yao J, Stewart ME, Lee TW, Gray SK, Nuzzo RG, Rogers JA. nanopost Plasmonic Crystals. Nanotechnology. 2009;20 doi: 10.1088/0957-4484/20/43/434011. 434011/1. [DOI] [PubMed] [Google Scholar]
- 16.Mines GA, Tzeng BC, Stevenson KJ, Li JL, Hupp JT. Microporous Supramolecular Coordination Compounds as Chemosensory Photonic Lattices. Angew Chem Int Edit. 2002;41:154–157. doi: 10.1002/1521-3773(20020104)41:1<154::aid-anie154>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 17.Bailey RC, Hupp JT. Large-Scale Resonance Amplification of Optical Sensing of Volatile Compounds with Chemoresponsive Visible-Region Diffraction Gratings. J. Am. Chem. Soc. 2002;124:6767–6774. doi: 10.1021/ja0177354. [DOI] [PubMed] [Google Scholar]
- 18.Wark AW, Lee HJ, Qavi AJ, Corn RM. Nanoparticle-Enhanced Diffraction Gratings for Ultrasensitive Surface Plasmon Biosensing. Anal. Chem. 2007;79:6697–6701. doi: 10.1021/ac071062b. [DOI] [PubMed] [Google Scholar]
- 19.Lee HJ, Wark AW, Corn RM. Enhanced Bioaffinity Sensing Using Surface Plasmons, Surface Enzymes Reactions, Nanoparticles and Diffraction Gratings. Analyst. 2008;133:596–601. doi: 10.1039/b718713k. [DOI] [PubMed] [Google Scholar]
- 20.Sendroiu IE, Corn RM. Nanoparticle Diffraction Gratings for DNA Detection on Photopatterned Glass Substrates. Biointerphases. 2008;3:FD23–FD29. doi: 10.1116/1.2994689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holtz JH, Asher SA. Polymerized Colloidal Crystal Hydrogel Films as Intelligent Chemical Sensing Materials. Nature. 1997;389:829–832. doi: 10.1038/39834. [DOI] [PubMed] [Google Scholar]
- 22.Li YY, Cunin F, Link JR, Gao T, Betts RE, Reiver SH, Chin V, Bhatia SN, Sailor MJ. Polymer Replicas of Photonic Porous Silicon for Sensing and Drug Delivery Applications. Science. 2003;299:2045–2047. doi: 10.1126/science.1081298. [DOI] [PubMed] [Google Scholar]
- 23.Scott RWJ, Yang SM, Chabanis G, Coombs N, Williams DE, Ozin GA. Adv. Mater. (Weinheim, Ger.) 2001;13:1468. [Google Scholar]
- 24.Lee YJ, Braun PV. Tunable Inverse Opal Hydrogel pH Sensors. Adv. Mater. (Weinheim, Ger.) 2003;15:563–566. [Google Scholar]
- 25.Pacholski C, Sartor M, Sailor MJ, Cunin F, Miskelly GM. Biosensing Using Porous Silicon Double-Layer Inteferometers: Reflective Interferometric Fourier Transform Spectroscopy. J. Am. Chem. Soc. 2005;127:11636–11645. doi: 10.1021/ja0511671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruminski AM, King BH, Salonen J, Snyder JL, Sailor MJ. Porous Silicon-Based Optical Microsensors for Volatile Organic Analytes: Effect of Surface Chemistry on Stability and Specificity. Adv. Funct. Mater. 2010;20:2874–2883. [Google Scholar]
- 27.Ruminski AM, Barillaro G, Chaffin C, Sailor MJ. Internally Referenced Remote Sensors for HF and Cl2 Using Reactive Porous Silicon Photonic Crystals. Adv. Funct. Mater. 2011;21:1511–1525. [Google Scholar]
- 28.Ruminski AM, Moore MM, Sailor MJ. Humidity-Compensating Sensor for Volatile Organic Compounds Using Stacked Porous Silicon Photonic Crystals. Adv. Funct. Mater. 2008;18:3418–3426. [Google Scholar]
- 29.Orosco MM, Pacholski C, Sailor MJ. Real-Time Monitoring of Enzyme Activity in a Mesoporous Silicon Double Layer. Nat. Nanotechnol. 2009;4:255–258. doi: 10.1038/nnano.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meade SO, Chen MY, Sailor MJ, Miskelly GM. Multiplexed DNA Detection Using Spectrally Encoded Porous SiO2 Photonic Crystal Particles. Anal. Chem. 2009;81:2618–2625. doi: 10.1021/ac802538x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park SH, Seo D, Kim YY, Lee KW. Organic Vapor Detection Using a Color-Difference Image Technique for Distributed Bragg Reflector Structured Porous Silicon. Sensors and Actuators B-Chemical. 2010;147:775–779. [Google Scholar]
- 32.Kim HJ, Kim YY, Lee KW. Sensing Characteristics of the Organic Vapors According to the Reflectance Spectrum in the Porous Silicon Multilayer Structure. Sensors and Actuators A-Physical. 2011;165:276–279. [Google Scholar]
- 33.Hidalgo N, Calvo ME, Miguez H. Mesostructured Thin Films as Responsive Optical Coatings of Photonic Crystals. Small. 2009;5:2309–2315. doi: 10.1002/smll.200900411. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Garcia L, Lozano G, Barranco A, Miguez H, Gonzalez-Elipe AR. TiO2-SiO2 One-Dimensional Photonic Crystals of Controlled Porosity by Glancing Angle Physical Vapour Deposition. J. Mater. Chem. 2010;20:6408–6412. [Google Scholar]
- 35.De Stefano L, Rotiroti L, De Tommasi E, Rea I, Rendina I, Canciello M, Maglio G, Palumbo R. Hybrid Polymer-Porous Silicon Photonic Crystals for Optical Sensing. J. Appl. Phys. 2009;106 023109. [Google Scholar]
- 36.Perelman LA, Moore T, Singelyn J, Sailor MJ, Segal E. Preparation and Characterization of a pH- and Thermally Responsive Poly(N-isopropylacrylamide-co-acrylic acid)/Porous SiO2 Hybrid. Adv. Funct. Mater. 2010;20:826–833. doi: 10.1002/adfm.200900822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobler J, Lotsch BV, Ozin GA, Bein T. Vapor-Sensitive Bragg Mirrors and Optical Isotherms from Meoporous Nanoparticle Suspensions. ACS Nano. 2009;3:1669–1676. doi: 10.1021/nn800911c. [DOI] [PubMed] [Google Scholar]
- 38.Redel E, Mirtchev P, Huai C, Petrov S, Ozin GA. Nanoparticle Films and Photonic Crystal Multilayers from Colloidally Stable, Size-Controllable Zinc and Iron Oxide Nanoparticles. ACS Nano. 2011;5:2861–2869. doi: 10.1021/nn103464r. [DOI] [PubMed] [Google Scholar]
- 39.Bonifacio LD, Puzzo DP, Breslav S, Willey BM, McGeer A, Ozin GA. Towards the Photonic Nose: A Novel Platform for Molecule and Bacterial Identification. Adv. Mater. (Weinheim, Ger.) 2010;22:1351–1354. doi: 10.1002/adma.200902763. [DOI] [PubMed] [Google Scholar]
- 40.Vollmer F, Braun D, Libchaber A, Khoshsima M, Teraoka I, Arnold S. Protein Detection by Optical Shift of a Resonant Microcavity. Appl. Phys. Lett. 2002;80:4057–4059. [Google Scholar]
- 41.Arnold S, Khoshsima M, Teraoka I, Holler S, Vollmer F. Shift of Whispering-Gallery Modes in Microspheres by Protein Adsorption. Opt. Lett. 2003;28:272–274. doi: 10.1364/ol.28.000272. [DOI] [PubMed] [Google Scholar]
- 42.Armani AM, Kulkarni RP, Fraser SE, Flagan RC, Vahala KJ. Label-Free, Single-Molecule Detection with Optical Microcavities. Science. 2007;317:783–787. doi: 10.1126/science.1145002. [DOI] [PubMed] [Google Scholar]
- 43.Hunt HK, Soteropulos C, Armani AM. Biocongujation Strategies for Microtoroidal Optical Resonators. Sensors-Basel. 2010;10:9317–9336. doi: 10.3390/s101009317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iqbal M, Gleeson MA, Spaugh B, Tybor F, Gunn WG, Hochberg M, Baehr-Jones T, Bailey RC, Gunn LC. Label-Free Biosensors Arrays Based on Silicon Ring Resonators and High-Speed Optical Scanning Instrumentation. IEEE J. Sel. Top. Quantum Electron. 2010;16:654–661. [Google Scholar]
- 45.Xu DX, Vachon M, Densmore A, Ma R, Delage A, Janz S, Lapointe J, Li Y, Lopinski G, Zhang D, Liu QY, Cheben P, Schmid JH. Label-Free Biosensor Array Based on Silicon-on-Insulator Ring Resonators Addressed Using a WDM Approach. Opt Lett. 2010;35:2771–2773. doi: 10.1364/OL.35.002771. [DOI] [PubMed] [Google Scholar]
- 46.Washburn AL, Luchansky MS, Bowman AL, Bailey RC. Quantitative, Label-Free Detection of Five Protein Biomarkers Using Multiplexed Arrays of Silicon Photonic Microring Resonators. Anal. Chem. 2010;82:69–72. doi: 10.1021/ac902451b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qavi AJ, Bailey RC. Multiplexed Detection and Label-Free Quantitation of MicroRNAs Using Arrays of Silicon Photonic Microring Resonators. Angew Chem Int Edit. 2010;49:4608–4611. doi: 10.1002/anie.201001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luchansky MS, Bailey RC. Silicon Photonic Microring Resonators for Quantitative Cytokine Detection and T-Cell Secretion Analysis. Anal. Chem. 2010;82:1975–1981. doi: 10.1021/ac902725q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Densmore A, Vachon M, Xu DX, Janz S, Ma R, Li YH, Lopinski G, Delage A, Lapointe J, Luebbert CC, Liu QY, Cheben P, Schmid JH. Silicon Photonic Wire Biosensor Array for Multiplexed Real-Time and Label-Free Molecular Detection. Opt Lett. 2009;34:3598–3600. doi: 10.1364/OL.34.003598. [DOI] [PubMed] [Google Scholar]
- 50.Janz S, Densmore A, Xu DX, Cheben P, Ma R, Schmid JH, Delage A, Vachon M, Lapointe J, Sabourin N, Sinclair W, Li Y, Mischki T, Lopinski G, MacKenzie R, Liu Q, Post E, Lamontagne B. Proc. SPIE. 2011;7888 788805. [Google Scholar]
- 51.Densmore A, Xu DX, Vachon M, Janz S, Ma R, Li Y, Lopinski G, Luebbert CC, Liu Q, Schmid JH, Delage A, Cheben P. Proc. of SPIE. 2010;7606 76060C. [Google Scholar]
- 52.Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold Nanoparticles for Biology and Medicine. Angew Chem Int Edit. 2010;49:3280–3294. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu Y, Liu JW. Catalyst-Functionalized Nanomaterials. Wiley Interdisciplinary Reviews-Nanomedicine and Nanobiotechnology. 2009;1:35–46. doi: 10.1002/wnan.21. [DOI] [PubMed] [Google Scholar]
- 54.De M, Ghosh PS, Rotello VM. Applications of Nanoparticles in Biology. Advanced Materials (Weinheim, Germany) 2008;20:4225–4241. [Google Scholar]
- 55.Suslick KS, Bailey DP, Ingison CK, Janzen M, Kosal ME, McNamara WB, III, Rakow NA, Sen A, Weaver JJ, Wilson JB, Zhang C, Nakagaki S. See Smells: Development of an Optoelectronic Nose. Quim. Nova. 2007;30:677–681. [Google Scholar]
- 56.Rakow NA, Suslick KS. A Colorimetric Sensor Array for Odour Visualization. Nature. 2000;406:710–713. doi: 10.1038/35021028. [DOI] [PubMed] [Google Scholar]
- 57.Suslick KS. An Optoelectronic Nose: "Seeing" Smells by Means of Colorimetric Sensor Arrays. MRS Bull. 2004;29:720–725. doi: 10.1557/mrs2004.209. [DOI] [PubMed] [Google Scholar]
- 58.Suslick KS, Rakow NA, Sen A. Colorimetric Sensor Arrays for Molecular Recognition. Tetrahedron. 2004;60:11133–11138. [Google Scholar]
- 59.Rakow NA, Sen A, Janzen MC, Ponder JB, Suslick KS. Molecular Recognition and Discrimination of Amines with a Colorimetric Array. Angew. Chem. Int. Ed. 2005;44:4528–4532. doi: 10.1002/anie.200500939. [DOI] [PubMed] [Google Scholar]
- 60.Zhang C, Suslick KS. A Colorimetric Sensor Array for Organics in Water. J. Am. Chem. Soc. 2005;127:11548–11549. doi: 10.1021/ja052606z. [DOI] [PubMed] [Google Scholar]
- 61.Janzen MC, Ponder JB, Bailey DP, Ingison CK, Suslick KS. Colorimetric Sensor Arrays for Volatile Organic Compounds. Anal. Chem. 2006;78:3591–3600. doi: 10.1021/ac052111s. [DOI] [PubMed] [Google Scholar]
- 62.Zhang C, Bailey DP, Suslick KS. Colorimetric Sensor Arrays for the Analysis of Beers: A Feasibility Study. J. Agric. Food Chem. 2006;54:4925–4931. doi: 10.1021/jf060110a. [DOI] [PubMed] [Google Scholar]
- 63.Zhang C, Suslick KS. Colorimetric Sensor Array for Soft Drink Analysis. J. Agric. Food Chem. 2007;55:237–242. doi: 10.1021/jf0624695. [DOI] [PubMed] [Google Scholar]
- 64.Bang JH, Lim SH, Park E, Suslick KS. Chemically Responsive Nanoporous Pigments: Colorimetric Sensor Arrays and the Identification of Aliphatic Amines. Langmuir. 2008;24:13168–13172. doi: 10.1021/la802029m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim SH, Kemling JW, Feng L, Suslick KS. A Colorimetric Sensor Array of Porous Pigments. Analyst (Cambridge, U. K.) 2009;134:2453–2457. doi: 10.1039/b916571a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jeronimo PCA, Araujo AN, Montenegro MCBSM. Optical Sensors and Biosensors Based on Sol-Gel Films. Talanta. 2007;72:13–27. doi: 10.1016/j.talanta.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 67.Podbielska H, Ulatowska-Jarza A, Muller G, Eichler HJ. Sol-Gels for Optical Sensors. Erice, Italy: Springer; 2006. [Google Scholar]
- 68.Avnir D. Organic-Chemistry within Ceramic Matrices -Doped Sol-Gel Materials. Acc. Chem. Res. 1995;28:328–334. [Google Scholar]
- 69.Kemling JW, Suslick KS. Nanoscale Porosity in Pigments for Chemical Sensing. Nanoscale. 2011;3:1971–1973. doi: 10.1039/c0nr00963f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lim SH, Feng L, Kemling JW, Musto CJ, Suslick KS. An Optoelectronic Nose for the Detection of Toxic Gases. Nature Chem. 2009;1:562–567. doi: 10.1038/nchem.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feng L, Musto CJ, Kemling JW, Lim SH, Suslick KS. A Colorimetric Sensor Array for Identification of Toxic Gases Below Permissible Exposure Limits. Chem. Commun. (Cambridge, U. K.) 2010;46:2037–2039. doi: 10.1039/b926848k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feng L, Musto CJ, Kemling JW, Lim SH, Zhong W, Suslick KS. Colorimetric Sensor Array for Determination and Identification of Toxic Industrial Chemicals. Anal. Chem. (Washington, DC, U. S.) 2010;82:9433–9440. doi: 10.1021/ac1020886. [DOI] [PubMed] [Google Scholar]
- 73.Suslick BA, Feng L, Suslick KS. Discrimination of Complex Mixtures by a Colorimetric Sensor Array: Coffee Aromas. Anal. Chem. (Washington, DC, U. S.) 2010;82:2067–2073. doi: 10.1021/ac902823w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lim SH, Musto CJ, Park E, Zhong W, Suslick KS. A Colorimetric Sensor Array for Detection and Identification of Sugars. Org. Lett. 2008;10:4405–4408. doi: 10.1021/ol801459k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Musto CJ, Lim SH, Suslick KS. Colorimetric Detection and Identification of Natural and Artificial Sweeteners. Anal. Chem. 2009;81:6526–6533. doi: 10.1021/ac901019g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wan Y, Shi YF, Zhao DY. Designed Synthesis of Mesoporous Solids via Nonionic-Surfactant-Templating Approach. Chemical Communications (Cambridge, United Kingdom) 2007:897–926. doi: 10.1039/b610570j. [DOI] [PubMed] [Google Scholar]
- 77.Lin HP, Mou CY. Structural and Morphological Control of Cationic Surfactant-Templated Mesoporous Silica. Accounts of Chemical Research. 2002;35:927–935. doi: 10.1021/ar000074f. [DOI] [PubMed] [Google Scholar]
- 78.Scott BJ, Wirnsberger G, Stucky GD. Mesoporous and Mesostructured Materials for Optical Applications. Chemistry of Materials. 2001;13:3140–3150. [Google Scholar]
- 79.Kim HJ, Lee SJ, Park SY, Jung JH, Kim JS. Detection of CuII by a Chemodosimeter-Functionalized Monolayer on Mesoporous Silica. Adv. Mater. (Weinheim, Ger.) 2008;20:3229–3234. [Google Scholar]
- 80.Kim E, Seo S, Seo ML, Jung JH. Functionalized Monolayers on Mesoporous Silica and on Titania Nanoparticles for Mercuric Sensing. Analyst (Cambridge, U. K.) 2010;135:149–156. doi: 10.1039/b915975d. [DOI] [PubMed] [Google Scholar]
- 81.El-Safty SA. Functionalized Hexagonal Mesoporous Silica Monoliths with Hydrophobic Azo-Chromophore for Enhanced CoII Ion Monitoring. Adsorption. 2009;15:227–239. [Google Scholar]
- 82.El-Safty SA, Ismail AA, Shahat A. Optical Supermicrosensor Responses for Simple Recognition and Sensitive Removal of CuII Ion Target. Talanta. 2011;83:1341–1351. doi: 10.1016/j.talanta.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 83.El-Safty SA. Organic-Inorganic Hybrid Mesoporous Monoliths for Selective Discrimination and Sensitive Removal of Toxic Mercury Ions. J. Mater. Sci. 2009;44:6764–6774. [Google Scholar]
- 84.Comes M, Aznar E, Moragues M, Marcos MD, Martinez-Manez R, Sancenon F, Soto J, Villaescusa LA, Gil L, Amoros P. Mesoporous Hybrid Materials Containing Nanoscopic "Binding Pockets" for Colorimetric Anion Signaling in Water by Using Displacemnt Assays. Chem.-A-Eur. J. 2009;15:9024–9033. doi: 10.1002/chem.200900890. [DOI] [PubMed] [Google Scholar]
- 85.Climent E, Casasus R, Marcos MD, Martinez-Manez R, Sancenon F, Soto J. Colorimetric Sensing of Pyrophosphate in Aqueous Media Using bis-Functionalized Silica Surfaces. Dalton Trans. 2009:4806–4814. doi: 10.1039/b902099c. [DOI] [PubMed] [Google Scholar]
- 86.Ros-Lis JV, Casasus R, Comes M, Coll C, Marcos MD, Martinez-Manez R, Sancenon F, Soto J, Amoros P, El Haskouri J, Garro N, Rurack K. A Mesoporous 3D Hybrid Material with Dual Functionality for Hg2+ Detection and Adsorption. Chem.-A-Eur. J. 2008;14:8267–8278. doi: 10.1002/chem.200800632. [DOI] [PubMed] [Google Scholar]
- 87.Comes M, Marcos MD, Martinez-Manez R, Sancenon F, Villaescusa LA, Graefe A, Mohr GJ. Hybrid Functionalised Mesoporous Silica-Polymer Composites for Enhanced Analyte Monitoring Using Optical Sensors. J. Mater. Chem. 2008;18:5815–5823. [Google Scholar]
- 88.Johnson-White B, Zeinali M, Shaffer KM, Patterson CH, Charles PT, Markowitz MA. Detection of Organics Using Porphyrin Embedded Nanoporous Organosilicas. Biosens. Bioelectron. 2007;22:1154–1162. doi: 10.1016/j.bios.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 89.Johnson BJ, Anderson NE, Charles PT, Malanoski AP, Melde BJ, Nasir M, Deschamps JR. Porphyrin-Embedded Silicate Materials for Detection of Hydrocarbon Solvents. Sensors-Basel. 2011;11:886–904. doi: 10.3390/s110100886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li F, Stein A. Functional Composite Membranes Based on Mesoporous Silica Spheres in a Hierarchically Porous Matrix. Chem. Mater. 2010;22:3790–3797. [Google Scholar]