Abstract
Network oscillations support transient communication across brain structures. We show here, in rats, that task-related neuronal activity in the medial prefrontal cortex (PFC), hippocampus and ventral tegmental area (VTA), regions critical for working memory, is coordinated by a 4-Hz oscillation. A prominent increase of power and coherence of the 4-Hz oscillation in PFC and VTA and its phase-modulation of gamma power in both structures was present in the working memory part of the task. Subsets of both PFC and hippocampal neurons predicted the turn choices of the rat. The goal-predicting PFC pyramidal neurons were more strongly phase-locked to both 4-Hz and hippocampal theta oscillations than non-predicting cells. The 4-Hz and theta oscillations were phase-coupled and jointly modulated both gamma waves and neuronal spikes in PFC, VTA and hippocampus. Thus, multiplexed timing mechanisms in the PFC-VTA-hippocampus axis may support processing of information, including working memory.
Introduction
The prefrontal cortex (PFC), with its abundant anatomical interconnections with numerous other cortical/subcortical areas, is thought to play a key role in the integration of information from different brain regions, and supports various cognitive functions (Fuster, 2001; Miller and Cohen, 2001). In particular, PFC is thought to be a pivotal substrate for maintaining information in the absence of changing external inputs, and neuronal activity in this brain region is assumed to be critical in working memory (Goldman-Rakic, 1995; Baddeley 2003). To implement memory related activity, the PFC has been suggested to work in synergy with other brain regions, including the basal ganglia and the hippocampus (Fuster, 2001; Miller and Cohen, 2001). It has been shown that recruitment of memory-active neurons in PFC depends on task-relevant dopamine release from ventral tegmental area (VTA) neurons (Williams and Goldman-Rakic, 2002; Watanabe et al., 1997; Lewis and O'Donnell 2000; Schultz, 2006). Another synergistic mechanism of PFC with other brain regions is implied by EEG studies in humans. These experiments have demonstrated that the power of EEG oscillations in the 3 to 7 Hz band, recorded from the scalp above the PFC area (called “midline frontal theta”), correlates with working memory performance (Gevins et al., 1997; Sauseng et al., 2010). In human studies, it has been tacitly assumed that the midline frontal theta rhythm is generated by the hippocampus (Klimesch et al., 2001; Canolty et al., 2006; Fuentemilla et al., 2010). In support of this hypothesis, recent experiments in rodents have shown increased phase-coupling between hippocampal theta oscillations (7-9 Hz) and PFC neuronal firing during the working memory aspects of spatial tasks (Siapas et al., 2005; Jones and Wilson, 2005; Benchenane et al., 2010; Sigurdsson et al., 2010). However, theta frequency oscillations in the PFC are conspicuously weak or absent (Siapas et al., 2005; Jones and Wilson, 2005; Sirota et al., 2008) and it is unclear how the mesolimbic dopaminergic system is involved in hippocampal-PFC coordination (Benchenane et al., 2010; Lisman and Grace, 2005). Despite recent progress, the mechanisms underlying the temporal coordination of cell assemblies in the PFC-VTA-hippocampal system have remained ambiguous (Lisman and Grace, 2005).
In this study, we performed simultaneous large-scale recordings of neuronal activities and local field potentials in the medial prefrontal cortex (mPFC), VTA and hippocampus of the rat during a working memory task. We found that a 4-Hz (2-5 Hz band) oscillation is dominant in PFC-VTA circuits and is phase-coupled to hippocampal theta oscillations when working memory is in use. Both local gamma oscillations and neuronal firing can become phase-locked to the 4-Hz oscillation. PFC neurons that predict the behavioral choice of the rat are more strongly co-modulated by both 4-Hz and theta rhythms than non-predicting neurons. We hypothesize that these multiplexed, temporal coupling mechanisms underlie the dynamic coalition of cell assemblies in the PFC-VTA-hippocampal system, supporting specific cognitive functions such as working memory.
Results
Neuronal activity was recorded in a cue-place matching working memory task (Fig. 1A; Fujisawa et al., 2008), which required rats to associate an odor cue (chocolate or cheese) presented in the start box with a spatial position of a reward (left or right arm of the T-maze). All rats performed the working memory task at high levels of performance (92.7%±5.0 correct, mean ± SD, n = 57 sessions in 7 rats) at the time of neurophysiological data collection. For recording LFP and neuronal spikes, silicon probes or wire tetrodes were placed in the PFC, hippocampus and VTA (Fig. 1B; Supplementary Fig. 1). A total of 1,526 mPFC, 607 CA1, and 539 VTA neurons were recorded in the seven rats during the task behavior. To examine location bias of the physiological data in the maze quantitatively, lap trajectories were linearized and represented parametrically as a continuous, one-dimensional line for each trial (total length, 230 cm), beginning with the odor sampling (nose-poking) location (position 0) and ending with the reward area (position 1; Fig. 1A).
Figure 1.
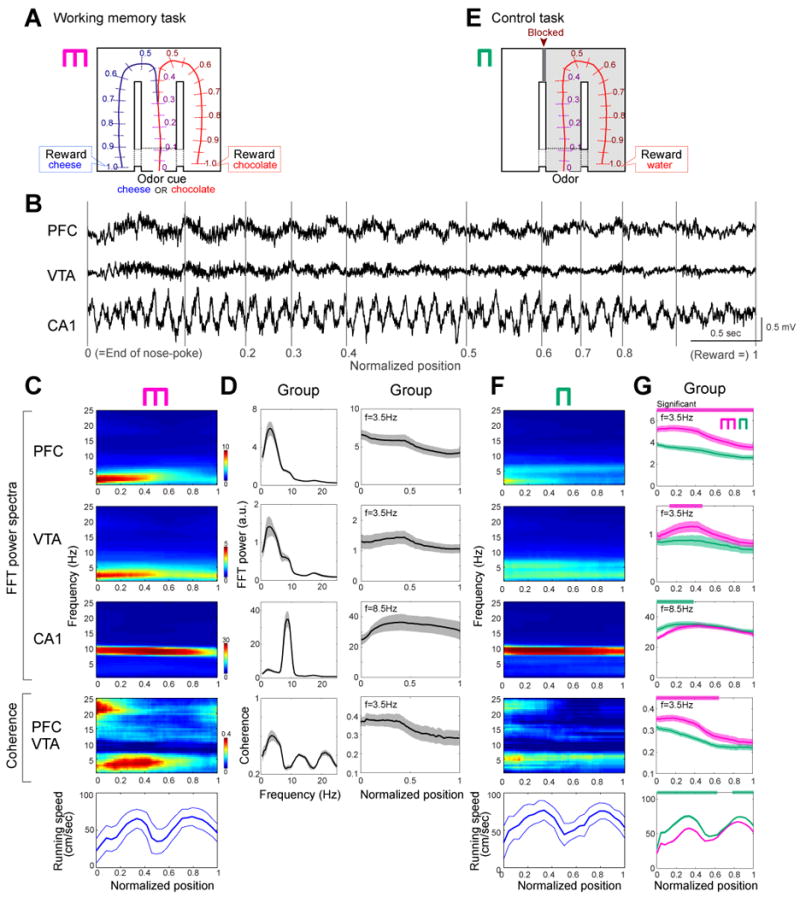
Enhanced power of the 4-Hz rhythm in PFC and VTA correlates with working memory. (A) Odor-based matching-to-sample task. An odor cue (chocolate or cheese) is presented following a nose-poke in a start box (position 0). Cheese or chocolate odor signals the availability of cheese or chocolate reward in the left or right goal area (position1), respectively. Lap trajectories were linearized and represented as a one-dimensional line. (B) LFP traces from PFC, VTA and the hippocampal CA1 pyramidal layer. Gray lines, normalized positions in the maze. (C) Time-resolved power (PFC, VTA, CA1) and coherence (PFC vs. VTA) spectra for rat M, as the function of position. Bottom, running speed (mean±SDM). To exclude spurious coupling, the baseline coherence values were calculated by shift predictor methods, and were subtracted from the absolute values (C-G). (D) Power spectra mean (±SEM) for all rats (left panels) and within-trial evolution of 4-Hz (VTA, PFC) or theta (CA1) power and PFC-VTA coherence (right panels) as the function of position. For these measurements the peak power at the 4-Hz or theta band was used. (E) Control task with the left arm blocked. (F) Time-resolved spectra in the control task, performed on the same day in (C). Color scales are same as (C). (G) Group comparisons between control (green) and working memory (magenta) tasks. The frequency bands used in these analyses are the same as in (D). Significant increases or decreases in the memory task are shown by magenta and green horizontal lines, respectively (P<0.01; permutation test; Fujisawa et al., 2008).
Task-modulated 4-Hz, theta and gamma oscillations
LFP patterns in the PFC, VTA and hippocampus were characteristically different, with a dominant 7-9 Hz theta oscillation in the hippocampus and a 2 to 5 Hz (‘4-Hz’ for short; 3.54 ± 0.63 Hz) oscillation in the PFC and VTA (Fig. 1B). The most prominent physiological change in the working memory task was the significantly larger 4-Hz power of the PFC and VTA signals and their coherence in the central (‘choice’) arm of the T maze (segments 0.0 to 0.5 in Fig. 1C, D), compared to the side arms (segments 0-0.3 vs. 0.6-1.0; P<0.01 for PFC, VTA, and PFC-VTA coherence; paired t-test). Left and right lap trajectories in segments 0 to 0.3 of the central arm overlapped and began to differ significantly at position 0.32±0.041 (P<0.01; permutation test; Fujisawa et al., 2008). Although the power of hippocampal theta oscillations (Fig. 1C, D; Supplementary Figs. 2) and theta band coherence between hippocampus and PFC/VTA (Supplementary Fig 2) were also high in the central arm, they remained elevated until the rat reached the goal location and consumed the reward. To exclude the possibility that elevated 4-Hz signal was simply reflected some motor, non-cognitive aspects, three of the 7 rats were also tested in a control, non-memory task (Fig. 1E; Methods). The power of the 4-Hz oscillation in both PFC and VTA was significantly lower in the control task than in the memory task (P<0.01 for PFC, P<0.05 for VTA; permutation test; Fujisawa et al., 2008), with the largest differences present in the central arm. The 4-Hz coherence between PFC and VTA signals was also significantly higher in the memory task compared to the control task (Fig. 1F, G; P<0.01; for individual rats, see Supplementary Fig. 2). To examine the possible confounding role of movement variables further, the running speed of the rats was correlated with the power of PFC 4-Hz and hippocampal theta oscillations. While hippocampal theta power was positively correlated with the velocity of the animals (P<0.01; McFarland et al., 1975), no such relationship was observed between the velocity and PFC 4-Hz power (P=0.3).
To exclude the potential confound of different odorants and reward magnitude expectancies, PFC and hippocampal activity patterns were also examined in a spontaneous alternation task in other three of the 7 rats. During the delay between trials, the animal was required to run in a wheel (Pastalkova et al., 2008) and rewarded with the same amount of water after choosing the correct left or right arm. The spontaneous alternation task is sensitive to both hippocampal and PFC lesions as well as to dopamine agonists (Divac et al., 1975; Stevens and Cowey, 1973). While the rat had to keep the information about the previous choice as working memory for an extended duration (i.e., during running in the wheel and the central arm), the power of 4-Hz oscillation was high throughout, whereas in the side arms it was significantly lower (Supplementary Fig.3; n=17 sessions; P<0.01, wheel vs. side arms; P<0.01; wheel vs. 0-0.3 segment of central arm; paired t-test), as in the cue-place matching working memory task. In contrast, hippocampal theta power was more strongly correlated with motor aspects of the task (Supplementary Fig. 3) than with working memory, again similar to the cue-place matching working memory task. These findings demonstrate that the power of 4-Hz oscillation in PFC and VTA and the coherence between these structures are correlated with the working memory component of the task.
Because the involvement of local circuits in a task is often reflected by gamma band oscillations (Canolty et al., 2006; Gray et al., 1989; Siegel et al., 2009), and because the power of gamma oscillations in PFC is correlated with working memory in humans (Howard et al., 2003), we next investigated the influence of the 4-Hz rhythm on gamma activity (30-80 Hz). A narrow-band gamma oscillation with a 50-Hz peak dominated in both PFC and VTA in the central arm (Fig. 2), and mimicked the dynamics of the 4-Hz power in both the cue-place matching working-memory and control tasks (Fig. 1C, F). Despite the large anatomical distance between PFC and VTA, gamma coherence between these structures was significantly high (Fig. 2C; P<0.01; permutation test), indicating that activity in PFC and VTA also synchronizes at short time-scales in a behaviorally dependent manner. In addition to gamma phase coherence, the amplitude of gamma waves (Fig. 3A) in both PFC and VTA was phase-modulated by the PFC 4-Hz oscillation, with the strongest modulation present in the central arm (Fig. 3A). In contrast, coherence between hippocampal LFP and the envelope of local gamma amplitude in different segments of the maze largely paralleled the power of the theta rhythm in the hippocampus (Fig. 3A), and co-varied more with the motoric aspects of the task than with the working memory component. To determine the phase at which the 4-Hz rhythm modulated gamma power, troughs of the filtered gamma waves were used to construct LFP averages from epochs corresponding to different locations of the rat (Fig. 3B). This analysis showed that the largest amplitude of gamma waves occurs on the ascending phase of the 4-Hz oscillation (preferred phase: 241.1°±11.8). Moreover, the largest amplitude and most strongly modulated average occurred in the central arm of the working memory task, compared with the side arm and averages obtained in the control task.
Figure 2.
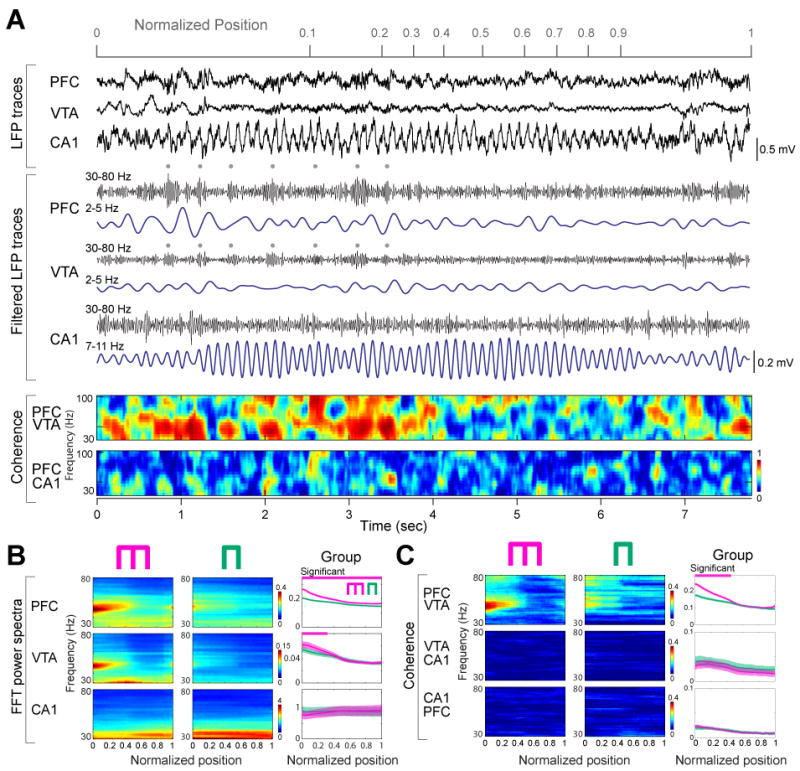
PFC-VTA gamma oscillation power and coherence are correlated with working memory. (A) Representative LFP traces and their filtered derivatives from PFC, VTA and the hippocampal CA1 pyramidal layer (rat M). Dots: 4-Hz rhythmic increase of gamma amplitude in PFC and VTA. Color plots, PFC-VTA and PFC-HPC coherence of gamma activity. (B) Time-resolved power spectra in the working memory and control sessions in a single session. Right, group comparisons between working memory (magenta) and control (green) tasks (n=29 sessions in 3 rats). Magenta line, significant group differences (P<0.01 for PFC, P<0.05 for VTA; permutation test; Fujisawa et al., 2008). (C) Time-resolved coherence spectra in the working memory and control sessions in a single rat, and in group (P<0.01; permutation test; spurious coupling subtracted).
Figure 3.
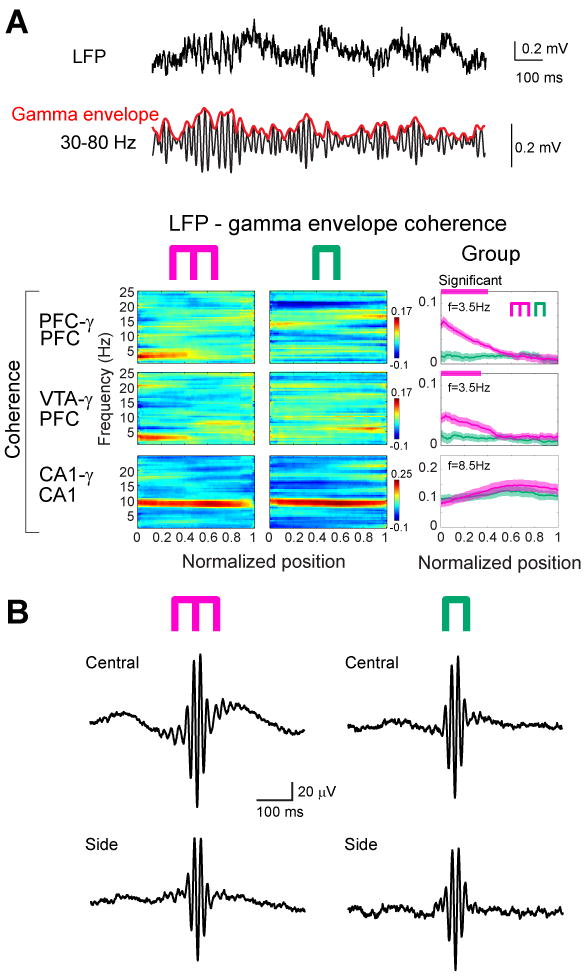
Working memory is correlated with 4-Hz modulation of gamma power. (A) Wideband (1 Hz-1250 Hz) and filtered (30-80 Hz) LFP from PFC. Red line indicates the envelope of gamma amplitude fluctuation. Color panels, time-resolved coherence spectra between the LFP and gamma envelope in the working memory and control sessions in a single rat. Right panels, group data of LFP vs gamma envelope coherence in the memory (magenta) and control (green) tasks. Note opposite changes of LFP vs gamma envelope in the 4-Hz and theta bands in both memory and control tasks. Horizontal magenta and green lines above panels, significant group differences (P<0.01 for PFC gamma, P<0.05 for VTA gamma; permutation test; Fujisawa et al., 2008; spurious coupling subtracted). (B) Gamma trough-triggered LFP in the central and side arms in both tasks. Note strongest phase-locking of gamma waves to the ascending phase 4-Hz oscillation in the central arm of the memory task (upper left).
Phase modulation of PFC, hippocampal and VTA neurons by 4-Hz and theta oscillations
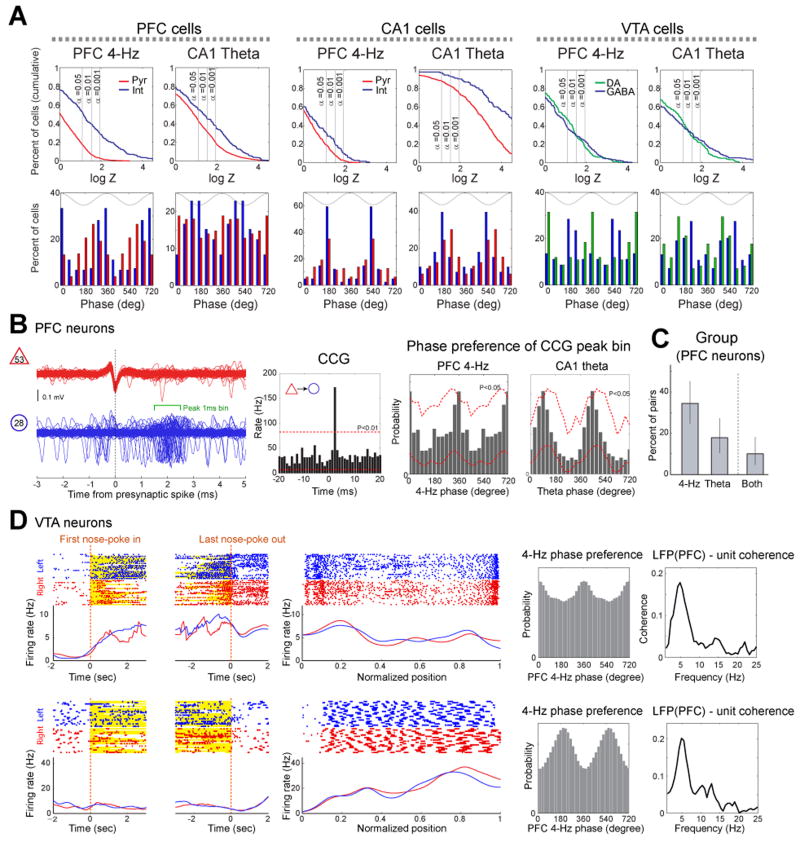
To gain insight about the local impact of the 4-Hz and theta oscillations on unit firing, their phase correlations with putative principal cells and interneurons were examined (Fig. 4A; Supplementary Fig. 4). A sizable fraction of neurons in both PFC and hippocampus was significantly modulated by the 4-Hz rhythm (PFC pyramidal cells: 17.7%, PFC interneurons: 51.6%; CA1 pyramidal cells: 17.8%, CA1 interneurons: 30.9%; P<0.05; Rayleigh test was used for assessing uniformity). Large percentages of neurons were also phase-locked to hippocampal theta oscillations (Fig. 4A; PFC pyramidal cells: 36.4%, PFC interneurons: 55.9% (Siapas et al., 2005; Sirota et al., 2008); CA1 pyramidal cells: 88.6%, CA1 interneurons: 96.8% (Sirota et al., 2008)). In addition to spike modulation, spike transmission efficacy between monosynaptically connected PFC neurons, as inferred from the short-term cross-correlograms between neuron pairs (Fig. 4B; Fujisawa et al., 2008), was also phase-modulated by both PFC 4-Hz and hippocampal theta oscillations in 42% and 22% of the pairs, respectively (Fig. 4C).
Figure 4.
Entrainment of unit firing by 4-Hz and theta oscillations. (A) Top, cumulative density function of phase modulation strength statistics logZ (Rayleigh statistics Z; Sirota et al., 2008) for putative pyramidal cells (red), GABAergic inhibitory cells (blue) and dopaminergic cells (green) from PFC (left), CA1 (middle) and VTA (right). Bottom, distribution of preferred phases for all significantly modulated (P<0.05) neurons. (B) Modulation of synaptic connections by 4-Hz and theta oscillations. Left, filtered traces of a synaptically connected PFC putative pyramidal cell (triangle)-interneuron (circle) pair and their cross-correlogram (CCG, second panel). Spike transmission efficacy was estimated by the short-term cross-correlograms between neuron pairs. Note short-latency (∼2ms) firing of the interneuron after the pyramidal cell spike in an example pair. Third and fourth panels, phase distribution of coincident spikes of the example pyramidal cell-interneuron pair (co-firing within 1-msec windows, 1 msec after pyramidal cell spike; Fujisawa et al., 2008) during PFC 4-Hz and hippocampal theta oscillations. Red lines: significance level estimated by permutation test (P<0.05; Fujisawa et al., 2008). (C) Percent of monosynaptically connected PFC pyramidal-interneuron pairs significantly modulated by either or both rhythms. Clopper-Pearson confidence intervals (P<0.05) are also shown. (D) Raster plots of unit firing and mean rates during left- (blue line) and right-bound (red line) trials for two representative VTA units related to nose poking onset (0 sec; left panel), offset (0 sec; 2nd panel) and activity (third panel) in the maze. Histogram: modulation of unit firing by PFC 4-Hz. Rightmost panel: Coherence between LFP in PFC and unit firing. Top unit shows increased firing rate at sniffing onset. Bottom unit fired rhythmic bursts of spikes during running (note rhythmicity of spikes).
Neurons in the VTA were classified as putative dopaminergic and putative GABAergic cells (Supplementary Figs. 4 and 5). Almost half of the putative dopaminergic (46.2%) and 37.5% of the putative GABAergic VTA neurons were significantly phase-locked to the 4-Hz oscillation, as shown by their phase histograms and the significant peaks in their unit-LFP coherence spectra (Fig. 4D). Approximately the same proportions of VTA neurons were also significantly phase-locked to the hippocampal theta rhythm (putative dopaminergic: 43.6%; putative GABAergic cells: 39.4%).
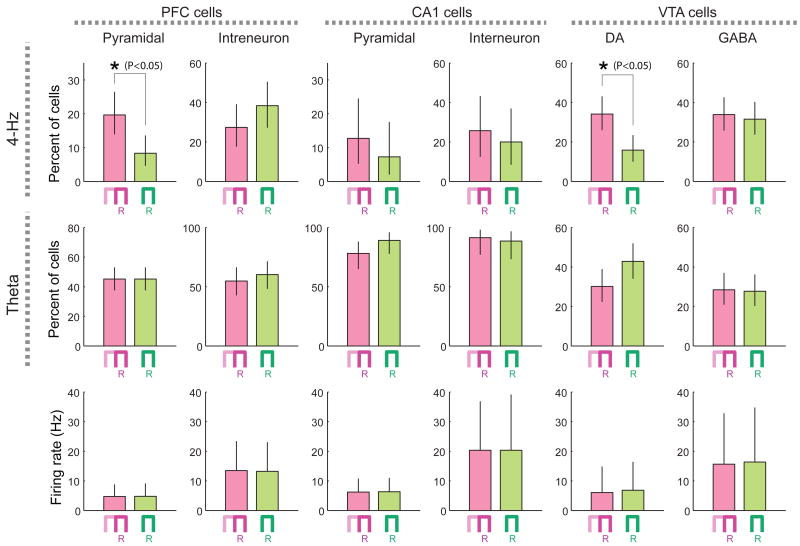
Phase-modulation of neurons by 4-Hz and theta oscillations was also compared between the memory and non-memory control tasks. For these comparisons, only the right-turn trials of the memory task were included and the same neurons were compared in both tasks. Despite the similar firing rates of the neurons in each brain area in the both tasks, almost twice as many PFC pyramidal cells and putative dopaminergic VTA neurons were significantly phase-locked to 4-Hz oscillation in the memory task than in the control task (Fig. 5). PFC interneurons, hippocampal cells and putative VTA GABAergic cells did not show such task-dependent phase-locking. The fraction of significantly theta phase-locked neurons in each brain region was similar in the two tasks (Fig. 5). These findings show that the magnitude of 4-Hz modulation of PFC pyramidal cells and VTA dopaminergic cells was task-specific.
Figure 5.
Phase-locking of unit firing to 4-Hz or theta oscillations in the working memory and control tasks. Percent of significantly phase-modulated PFC, CA1 and VTA cells (P<0.05; Rayleigh test) in the working memory (magenta) and control (green) tasks are shown. In these comparisons, only the right-turn trials of the memory task were included. Only neurons whose mean firing rates were > 1 Hz in the both tasks were included in the analyses. Bars, Clopper-Pearson confidence intervals (P < 0.05). Bottom, mean firing rates of the neurons (mean±SDM).
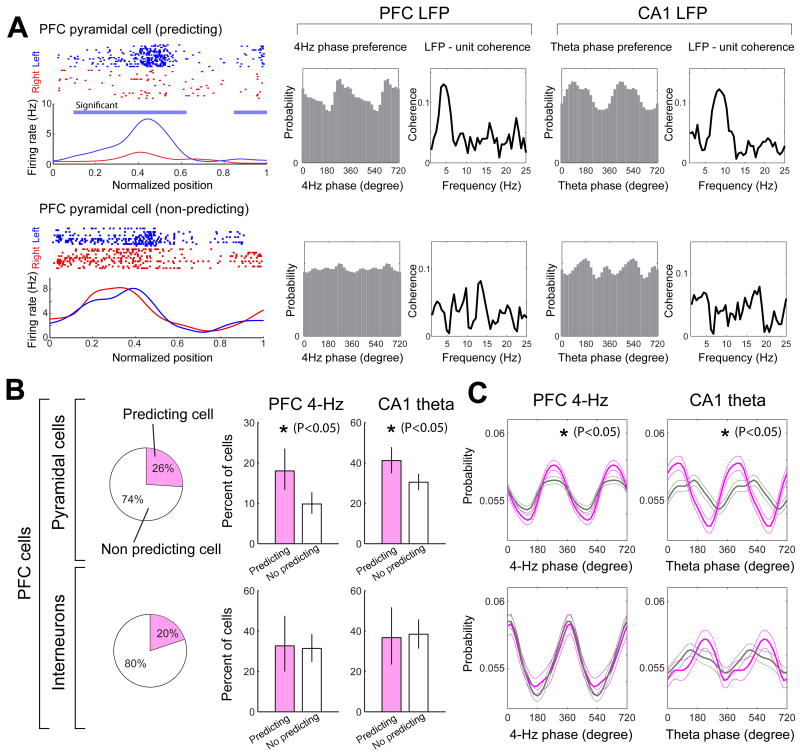
Enhanced modulation of goal-predicting PFC neurons by 4-Hz and theta oscillations
Although changes in 4-Hz and gamma oscillations in both PFC and VTA were reliably correlated with the working memory component of the task, they did not predict the direction choice of the animal on a given trial (P>0.05 for each rat). In contrast, a sizeable fraction of both PFC and hippocampal cells fired at significantly different rates in the central arm on trials with future left and right turns (Fig. 6A). Since such goal-predicting, ‘prospective’ or ‘episode’ neurons have been suggested to be a part of the working memory network (Wood et al., 2000; Frank et al., 2000; Fujisawa et al., 2008; Pastalkova et al., 2008), we examined their relationship with LFP oscillations and compared them with non-predicting but active neurons in the 0-0.3 segment of the central arm, where movement trajectories and speed during left and right trials were indistinguishable (Fujisawa et al., 2008). For these comparisons, only neurons which fired at least 1 Hz in the central arm were included. The fraction of both 4-Hz and theta-modulated neurons was significantly higher in the goal-predicting PFC pyramidal cell group (Fujisawa et al., 2008), compared to non-predicting cells (Fig. 6B). Furthermore, the magnitude of phase-locking of the goal-predicting PFC pyramidal cells to 4-Hz and theta oscillations was also significantly higher than phase-locking of the non-predicting neurons (Fig. 6C). This difference could not be explained by the significantly lower firing rate of non-predicting neurons in the central arm because the phase-modulation differences persisted after equalizing the spike numbers of all predicting and non-predicting neurons for the analysis, using exhaustive bootstrapping (Vinck et al., 2010; Supplementary Fig. 6). Although the fraction of predicting neurons in the hippocampus and PFC was similar, hippocampal predicting and non-predicting neurons did not show differential phase-locking to 4-Hz or theta oscillations (Supplementary Fig. 6).
Figure 6.
Trial outcome-predicting PFC neurons are more strongly modulated by the 4-Hz oscillation than non-predicting cells. (A) Raster plots and mean firing rates for two example PFC neurons during left (blue) and right-bound (red) trials. Blue horizontal line: significant difference (P<0.05; permutation test; Fujisawa et al., 2008) between left and right trials. Top row, side-predicting neuron with strong unit-LFP coherence at 4-Hz. Bottom, non-predicting neuron with poor unit-LFP coherence. (B) Pie charts, fractions of side-predicting (pink; significantly different mean discharge rates at position 0-0.3 in left and right trials; P<0.05) and non-predicting (white) neurons. Graphs, percentage of PFC neurons significantly phase-locked to PFC 4-Hz or hippocampal theta oscillations in the central arm. Clopper-Pearson confidence intervals (P < 0.05) are shown. (C) Mean phase modulation of predicting (pink) and non-predicting (gray) PFC neurons by 4-Hz and theta oscillations. Peak-to-trough height of the phase histograms was used for the assessment of statistical significance (P<0.05; permutation test).
Joint phase-modulation of PFC and hippocampal neurons by 4-Hz and theta oscillation
Since timing of neuronal spikes was biased by both 4-Hz and theta rhythms, we also examined their joint effects. First, we tested whether there is a phase-relationship between these oscillations. PFC 4-Hz trough (180°) was significantly locked to the trough (198.5±3.78°) of CA1 theta waves in each rat (Fig. 7A; P<0.01; shift predictor statistics). The phase-relationship between these oscillations was also indicated by single unit data. A fractions of neurons were significantly modulated by both PFC 4-Hz and hippocampal theta oscillations (Fig. 7B; PFC: 13.7 %, CA1: 21.0%, VTA: 16.9 %; P<0.05; Rayleigh test). Plotting all significantly jointly modulated neurons showed that the population of phase-locked units occupied a diagonal (Fig. 7C), similar to the joint phase distribution of 4-Hz and theta oscillations (Fig. 7A, second panel). The diagonal distribution of the co-modulation values is an indication of the interdependent nature of neuronal phase-locking of to both rhythms. Comparison between jointly modulated predicting and non-predicting PFC pyramidal neurons revealed that predicting neurons were significantly more strongly co-modulated by these rhythms than non-predicting cells (Fig. 7D; P<0.05; Supplementary Fig. 6). In addition, we found that local gamma oscillations in PFC and hippocampus were modulated by both PFC 4-Hz and CA1 theta oscillations (Supplementary Fig. 7). These findings suggest that PFC neurons, active in the working memory part of the task, are temporal coordinated (Jones and Wilson 2005; Benchenane et al., 2010; Rutishauser et al., 2010) by 4-Hz and theta oscillations. The expected results of such coordination is that the synchronously discharging predicting cells can exert a stronger impact on downstream targets that guide behavior, compared to the less synchronous non-predicting population.
Figure 7.
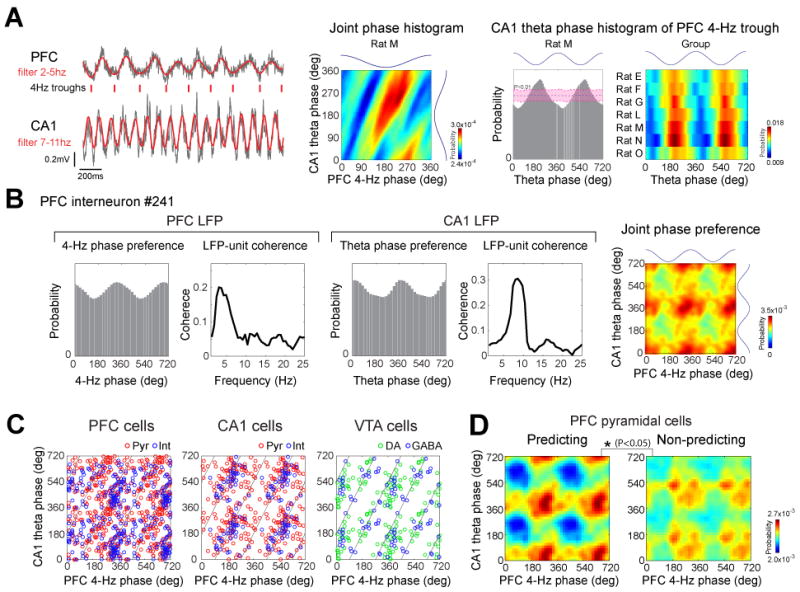
Joint modulation of neurons by 4-Hz and theta oscillations. (A) Phase relationship between 4-Hz and theta oscillations. Ticks indicate troughs of the filtered 4-Hz rhythm. Middle panel, joint phase histogram between PFC 4-Hz and hippocampal theta oscillations. Note diagonal bands indicating phase coupling. Right, theta phase histogram referenced to the trough of the 4-Hz oscillations for rat M and each rat (group; P<0.01; shift predictor statistics). Trough of waves is 180°. (B) Joint modulation of an example PFC neuron by both 4-Hz and theta oscillations. Unit firing histograms and coherence plots between unit discharge and PFC (left) and CA1 (right) LFP. Color plot: co-modulation of the neuron by both 4-Hz and theta oscillations. (C) Peaks of joint phase preferences for single neurons significantly phase-locked to both 4-Hz and theta oscillations in each structure. Diagonal lines, ideal trough-to-trough phase-coupling between 4-Hz and theta (8 Hz) oscillations. (D) Mean joint phase modulation of side-predicting and non-predicting neurons. Peak-to-trough height of the joint phase histograms was used for the assessment of statistical significance (P<0.05; permutation test).
4-Hz and theta are distinct oscillations
Finally, we compared LFP activity in PFC and hippocampus during task behaviors and in the home cage during waking immobility, REM sleep and slow wave sleep (Supplementary Fig. 8). PFC 4-Hz power was high during nose-poking, running in the central arm and wheel, i.e., during times when working memory was active. PFC 4-Hz power was low during immobility and sleep, including theta-dominated REM sleep. Hippocampal theta power was high during running behavior and REM-sleep, while low during nose-poking, immobility and slow wave sleep (Supplementary Fig. 8). Slow wave sleep in both PFC and hippocampus was dominated by a large 2-Hz peak, a reflection of slow oscillation of non-REM sleep (Steriade et al., 1993). This behavior-dependent dissociation of power changes demonstrates that theta and 4-Hz oscillations are distinct rhythms with characteristically different behavioral correlates, and presumably different mechanisms.
Discussion
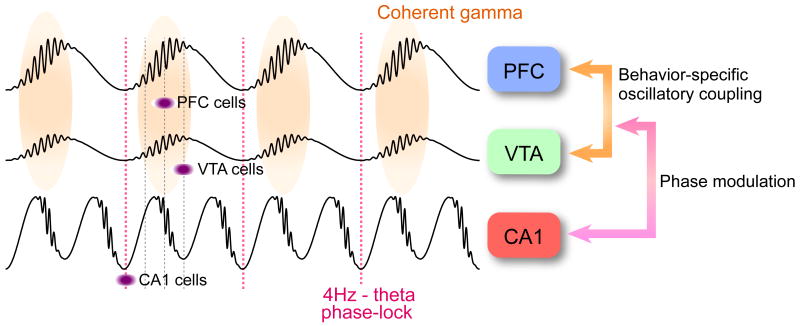
Our findings demonstrate a triple time control of neurons in the PFC-VTA-hippocampus axis by 4-Hz and theta rhythms at a slower time scale and gamma oscillations at a fine time scale (Fig. 8). The 4-Hz rhythm is the dominant pattern in PFC-VTA circuits, effectively modulating both local gamma oscillations and neuronal firing, whereas synchrony of neuronal spikes in the hippocampus is largely under the control of theta oscillations. Through phase-coupling, 4-Hz and theta oscillations jointly coordinate gamma oscillations and neuronal assembly patterns in a task-relevant manner. We hypothesize that these physiological mechanisms temporally coordinate neuron populations within and across the prefrontal, limbic and basal ganglia systems.
Figure 8.
A 4-Hz oscillation temporally coordinates activity across the limbic and basal ganglia systems. In PFC-VTA circuits, the 4-Hz rhythm is the dominant pattern, enhancing both coherent gamma oscillations and neuronal firing on the ascending phase. Through phase-coupling (red dotted line), 4-Hz and theta oscillations jointly coordinate gamma oscillations and neuronal assembly patterns according to task demands.
Coupling of PFC and VTA circuits by 4-Hz and gamma oscillations
Our observation of the prominent 4-Hz rhythm in PFC lead us to investigate the LFP and unit firing activity in VTA because of the prominent 2-5 Hz oscillatory firing patterns of dopaminergic neurons both in vitro and in vivo (Hyland et al., 2002; Paladini and Tepper, 1999; Bayer et al., 2007; Dzirasa et al., 2010; Kobayashi and Schultz 2008; see also our Fig. 4). Whereas the ‘pacemaker’ role of VTA is compatible with our observations, future experiments are needed to support this idea. Furthermore, even if dopaminergic neurons or the VTA circuit proves to be the fundamental source of the 4-Hz rhythm, it remains to be explained how the VTA entrains its target structures.
One possibility is that the 2-5 Hz rhythmic firing patterns of VTA dopaminergic neurons are transmitted through fast glutamatergic signaling to the target neurons (Koos et al., 2011). Recently, support for the co-release of glutamate and dopamine in the axon terminal of VTA dopaminergic neurons (Chuhma et al., 2004) has been reported in prefrontal cortex (Lavin et al., 2005; Yamaguchi et al., 2011). Another possibility is that the 3-6 Hz rhythm of dopaminergic neurons arises from the interaction with GABAergic neurons, since the blockade of GABAA receptors of dopaminergic neurons abolishes their 2-5 Hz firing pattern in vivo (Paladini and Tepper, 1999). Under the latter scenario, the 4-Hz activity can be broadcasted by the GABAergic neurons with projections to PFC (Carr and Sesack, 2000a).
Another striking observation of the present experiments is the task-dependent increase of gamma coherence between the PFC and VTA. Given the short period of the gamma rhythm, phase-coupling in this temporal range requires fast conduction mechanisms. A possible mechanism for such highly efficient coupling is a downstream PFC projection that is known to terminate on GABAergic neurons of the VTA (Carr and Sesack, 2000b). The preferential discharge of the putative GABAergic VTA neurons on the ascending phase of the 4-Hz rhythm provides support to this hypothesis. The return GABAergic projection from VTA to PFC (Carr and Sesack, 2000a) could also contribute to this fast signaling.
The presence of 4-Hz oscillations is visible in a number of previous reports even though the authors may have not emphasized them. Clear 3-6 Hz rhythmic activity was visible in the striatal recordings of mice during level pressing (Jin & Costa 2010) and in rats during ambulation or exploration (Tort et al., 2008; Berke et al., 2004; Dzirasa et al., 2011). The presence of theta wave ‘skipping’ of neurons (i.e., firing on every second theta cycle), firing rhythmically at 4-Hz, has been reported in deeper regions of the medial entorhinal cortex (Deshmukh et al., 2010). Similar theta wave skipping was observed in ventral hippocampal pyramidal neurons, resulting in a 4-Hz peak of their auto-correlograms (Royer et al., 2010). These observations and the present findings illustrate that 4-Hz oscillations are widespread and likely play a critical role in the temporal coordination of neuronal networks in the basal ganglia-PFC axis.
Interactions between 4-Hz and hippocampal theta oscillations
Several previous studies have demonstrated theta-coupling of PFC neurons in working memory tasks (Siapas et al., 2005; Jones and Wilson, 2005; Hyman et al., 2005; Benchenane et al., 2010). In addition to PFC, we found that a significant portion of VTA neurons were also phase-locked to theta, expanding the realm of theta oscillations to the mesolimbic dopamine system. The anatomical substrate and physiological mechanisms responsible for the theta entrainment of VTA cells remain to be identified. Theta phase-locked PFC neurons can, in principle, convey the theta rhythm to VTA GABAergic neuron (Carr and Sesack, 2000b). An alternative route is a polysynaptic pathway including the subiculum, nucleus accumbens and ventral pallidum. This indirect path has been suggested to carry novelty-induced signals from the hippocampus to the reward neurons in VTA (Lisman and Grace, 2005). The third possible pathway is the CA3-lateral septum-VTA projection (Luo et al., 2011). In return, VTA neurons can affect theta oscillations by their monosynaptic connections to the septal area (Gaykema and Záborszky, 1996) and hippocampus (cf., Lisman and Grace, 2005). In support for a role of the dopaminergic system in theta oscillations, transient inactivation of VTA decreases hippocampal theta power (Yoder and Pang, 2005) and VTA stimulation increases theta burst firing of medial septal neurons, mediated by D1/5 receptors (Fitch et al., 2006). Accordingly, the VTA, with its spontaneously oscillating neurons at 4 Hz, and the theta pacemaker medial septal area may form an interactive circuit, an ideal substrate for cross-frequency phase-coupling between the 4-Hz and theta rhythms.
Relationship between 4-Hz rhythm and midline frontal theta oscillations
The working memory component of the task in our experiments was correlated with the sustained power of 4-Hz oscillation and the phase-modulation of both gamma power and goal predicting PFC neurons by the 4-Hz rhythm. Power increase in the 3-8 Hz band near the frontal midline area of the scalp is the dominant EEG pattern during various cognitive tasks in humans, known as ‘frontal midline theta” (fm-theta; Gevins et al., 1997; for a review, see Mitchell et al., 2008; Sauseng et al., 2010). Two controversial issues of fm-theta have persisted: its specific behavioral correlates and the source of the fm-theta signal. Numerous studies have reported increased power of fm-theta during working memory tasks (Gevins et al., 1997; Sarnthein et al., 1998; Klimesch 2001; Onton et al., 2005). Intracranial recordings in patients also demonstrate a correlation between theta power and working memory (Raghavachari et al., 2001; Canolty et al., 2006). In contrast, other studies emphasize that fm-theta is best correlated with ‘mental concentration’ (Mizuki 1987; Gevins et al., 1997; Onton et al., 2005) or focused attention (Basar-Eroglu et al., 1992). This controversy may be explained by the co-occurrence of two rhythms in PFC, as shown here. In human studies, it is often tacitly assumed that the ‘cognitive frontal rhythm’ (Klimesch et al., 2001) is driven by hippocampal theta oscillations (Jensen and Tesche, 2002). Alternatively, it may be more related to the 4-Hz oscillations described here. In support of this hypothesis, several studies have pointed out that the behavioral specificity of mf-theta depends on the frequency band chosen (Klimesch 2001; Onton et al., 2005; Sauseng et al., 2010). Finally, recent studies in primates suggest that resetting of LFP in this low frequency band play an important role in attention and stimulus selection (Lakatos et al., 2008). We hypothesize that, similar to our observations in the PFC of the rat, two distinct oscillations with complementary roles are activated during working memory in the human prefrontal cortex and other mammals.
Conclusions
Structures within the limbic area and basal ganglia form distinct systems and perform different types of computations. However, systems often interact to support various behaviors. The representation strengths of memories and planning (served by PFC-hippocampus circuits) are often strongly affected by associated values (served by basal ganglia circuits; Luo et al., 2011). The various structures of the limbic system are bound together functionally by theta oscillations (Buzsaki, 2002). Our findings, along with previous observations by others, suggest that activity in the basal ganglia is temporally coordinated by a 4-Hz oscillation. We hypothesize that phase-phase (2:1) coupling mechanism between theta and 4-Hz oscillations provides a communication link between the limbic and basal ganglia systems (Fig. 8). Theta and 4-Hz oscillations can exert cross-structure, cross-frequency phase coupling effects on local operations, as reflected by the co-modulation of gamma power by these slower rhythms. In our experiments, the cross-frequency phase coupling effectively modulated task-specific PFC neurons, which carried goal-related positional and memory information. These findings illustrate how three independent rhythms (i.e., 4-Hz, theta and gamma) can coalesce transiently to perform specific actions. We hypothesize that phase coupling between the 4-Hz and theta oscillators, and their joint modulation of local gamma oscillations, may be a mechanism for linking the entorhinal-hippocampal spatial-contextual system with the mesolimbic dopaminergic reward system.
Methods
All protocols were approved by the Institutional Animal Care and Use Committee of Rutgers University. Seven adult male (3-5 months old) rats were trained in an odor-based delayed match-to-sample working memory task prior to surgery. The training apparatus was a figure 8 T-maze with a start area, where the sample odors (chocolate or cheese) were presented, and goal arms, which contained the reward. The animals were required to nose poke into a hole in the start box and the cue odor was given. If the cue was cheese odor, a piece of cheese (300mg) was given at the end of the right arm as reward. If the cue was chocolate, the reward was a piece of chocolate (300mg) at the left. The odor-side arm match varied across rats. Seven rats with a performance better than 85% correct choices in 5 consecutive days were chosen for surgery. Three out of the seven rats were also trained in a control, non-memory task. The left arm of the maze was blocked at the choice point, so that the animals could only enter the right arm and was always rewarded with a drop of water. To initiate a trial in the control task, the rats were required to nose-poke while coconut odor as presented. The control (non-memory) task was always followed by the working memory task after a 1∼2 hour rest in the home cage. For recording LFP and neuronal spikes, rats were implanted with silicon probes or tetrodes in the PFC, hippocampus CA1, and VTA (PFC-CA1 double recordings in 3 rats, PFC-VTA-CA1 triple recording in 4 rats; Fig. 1B; Supplementary Fig. 1). See Supplementary Information for further details.
Supplementary Material
Acknowledgments
We thank A. Amarasingham for help with data analysis and M. Belluscio, K. Diba, K. Mizuseki, J. Patel, A. Peyrache, E. Stark and D. Sullivan for comments on the manuscript. Supported by grants from the NIH (NS34994, MH54671), James S. McDonnell Foundation, NSF TDLC, the Uehara Memorial Foundation, the Naito Foundation, and the Japan Society for the Promotion of Science (S.F.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Basar E, Demiralp T, Schurmann M. P300-response: possible psychophysiological correlates in delta and theta frequency channels. A review Int J Psychophysiol. 1992;13:161–179. doi: 10.1016/0167-8760(92)90055-g. [DOI] [PubMed] [Google Scholar]
- Bayer HM, Lau B, Glimcher PW. Statistics of midbrain dopamine neuron spike trains in the awake primate. J Neurophysiol. 2007;98:1428–1439. doi: 10.1152/jn.01140.2006. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron. 2010;66:921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. 2004;43:883–96. doi: 10.1016/j.neuron.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000a;38:114–123. doi: 10.1002/1098-2396(200011)38:2<114::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000b;15:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Zhang H, Masson J, Zhuang X, Sulzer D, Hen R, Rayport S. Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses. J Neurosci. 2004;24:972–981. doi: 10.1523/JNEUROSCI.4317-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh SS, Yoganarasimha D, Voicu H, Knierim JJ. Theta modulation in the medial and the lateral entorhinal cortices. J Neurophysiol. 2010;104:994–1006. doi: 10.1152/jn.01141.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divac I, Wikmark RGE, Gade A. Spontaneous alternation in rats with lesions in the frontal lobes: an extension of the frontal lobe syndrome. Physiol Psychol. 1975;3:39–42. [Google Scholar]
- Dzirasa K, Coque L, Sidor MM, Kumar S, Dancy EA, Takahashi JS, McClung CA, Nicolelis MA. Lithium ameliorates nucleus accumbens phase-signaling dysfunction in a genetic mouse model of mania. J Neurosci. 2010;30:16314–16323. doi: 10.1523/JNEUROSCI.4289-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch TE, Sahr RN, Eastwood BJ, Zhou FC, Yang CR. Dopamine D1/5 receptor modulation of firing rate and bidirectional theta burst firing in medial septal/vertical limb of diagonal band neurons in vivo. J Neurophysiol. 2006;95:2808–2820. doi: 10.1152/jn.01210.2005. [DOI] [PubMed] [Google Scholar]
- Frank LM, Brown EN, Wilson M. Trajectory Encoding in the Hippocampus and Entorhinal Cortex. Neuron. 2000;27:169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- Fuentemilla L, Penny WD, Cashdollar N, Bunzeck N, Düzel E. Theta-coupled periodic replay in working memory. Curr Biol. 2010;20:606–612. doi: 10.1016/j.cub.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa S, Amarasingham A, Harrison MT, Buzsáki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nature Neurosci. 2008;11:823–834. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. London: Academic Press; 2001. [Google Scholar]
- Gaykema RP, Záborszky L. Direct catecholaminergic-cholinergic interactions in the basal forebrain II Substantia nigra-ventral tegmental area projections to cholinergic neurons. J Comp Neurol. 1996;374:555–577. doi: 10.1002/(SICI)1096-9861(19961028)374:4<555::AID-CNE6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gray CM, König P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;15:1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Jin X, Costa RM. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature. 2010;466:457–462. doi: 10.1038/nature09263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Yonelinas A, Kroll NE, Lazzara M, Rohm D, Gruber W. Theta synchronization during episodic retrieval: neural correlates of conscious awareness. Brain Res Cogn Brain Res. 2001;12:33–38. doi: 10.1016/s0926-6410(01)00024-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Schultz W. Influence of reward delays on responses of dopamine neurons. J Neurosci. 2008;28:7837–7846. doi: 10.1523/JNEUROSCI.1600-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tecuapetla F, Tepper JM. Glutamatergic signaling by midbrain dopaminergic neurons: recent insights from optogenetic, molecular and behavioral studies. Curr Opin Neurobiol. 2011;21:393–401. doi: 10.1016/j.conb.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- Lavin A, Nogueira L, Lapish CC, Wightman RM, Phillips PE, Seamans JK. Mesocortical dopamine neurons operate in distinct temporal domains using multimodal signaling. J Neurosci. 2005;25:5013–5023. doi: 10.1523/JNEUROSCI.0557-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BL, O'Donnell P. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential ‘up’ states in pyramidal neurons via D(1) dopamine receptors. Cereb Cortex. 2000;10:1168–1175. doi: 10.1093/cercor/10.12.1168. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland WL, Teitelbaum H, Hedges EK. Relationship between hippocampal theta activity and running speed in the rat. J Comp Physiol Psych. 1975;88:324–328. doi: 10.1037/h0076177. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitchell DJ, McNaughton N, Flanagan D, Kirk IJ. Frontal-midline theta from the perspective of hippocampal “theta”. Prog Neurobiol. 2008;86:156–185. doi: 10.1016/j.pneurobio.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Mizuki Y. Frontal lobe: Mental function and EEG. Am J EEG Technol. 1987;27:91–101. [Google Scholar]
- Montgomery SM, Sirota A, Buzsáki G. Theta and Gamma Coordination of Hippocampal Networks during Waking and Rapid Eye Movement Sleep. J Neurosci. 2008;28:6731–6741. doi: 10.1523/JNEUROSCI.1227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onton J, Delorme A, Makeig S. Frontal midline EEG dynamics during working memory. NeuroImage. 2005;27:341–356. doi: 10.1016/j.neuroimage.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Tepper JM. GABAA and GABAB antagonists differentially affect the firing pattern of substantia nigra dopaminergic neurons in vivo. Synapse. 1999;32:165–176. doi: 10.1002/(SICI)1098-2396(19990601)32:3<165::AID-SYN3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Lisman JE. Gating of human theta oscillations by a working memory task. J Neurosci. 2001;21:3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Sirota A, Patel J, Buzsáki G. Distinct representations and theta dynamics in dorsal and ventral hippocampus. J Neurosci. 2010;30:1777–1787. doi: 10.1523/JNEUROSCI.4681-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U, Ross IB, Mamelak AN, Schuman EM. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature. 2010;464:903–907. doi: 10.1038/nature08860. [DOI] [PubMed] [Google Scholar]
- Sarnthein J, Petsche H, Rappelsberger P, Shaw GL, von Stein A. Synchronization between prefrontal and posterior association cortex during human working memory. Proc Natl Acad Sci USA. 1998;95:7092–7096. doi: 10.1073/pnas.95.12.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Griesmayr B, Freunberger R, Klimesch W. Control mechanisms in working memory: a possible function of EEG theta oscillations. Neurosci Biobehav Rev. 2010;34:1015–1022. doi: 10.1016/j.neubiorev.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsáki G. Entrainment of neocortical neurons and gamma oscillations by hippocampal theta rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral Theories and the Neurophysiology of Reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Siegel M, Warden MR, Miller EK. Phase-dependent neuronal coding of objects in short-term memory. Proc Natl Acad Sci USA. 2009;106:21341–21346. doi: 10.1073/pnas.0908193106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Stevens R, Cowey A. Effects of dorsal and ventral hippocampal lesions on spontaneous alternation, learned alternation and probability learning in rats. Brain Res. 1973;52:203–224. doi: 10.1016/0006-8993(73)90659-8. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Kramer MA, Thorn C, Gibson DJ, Kubota Y, Graybiel AM, Kopell NJ. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc Natl Acad Sci USA. 2008;105:20517–20522. doi: 10.1073/pnas.0810524105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M, van Wingerden M, Womelsdorf T, Fries P, Pennartz CM. The pairwise phase consistency: a bias-free measure of rhythmic neuronal synchronization. Neuroimage. 2010;51:112–122. doi: 10.1016/j.neuroimage.2010.01.073. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kodama T, Hikosaka K. Increase of extracellular dopamine in primate prefrontal cortex during a working memory task. J Neurophysiol. 1997;78:2795–2798. doi: 10.1152/jn.1997.78.5.2795. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine Dl receptors in prefrontal cortex. Nature. 2002;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Li X, Ng TH, Morales M. Mesocorticolimbic glutamatergic pathway. J Neurosci. 2011;31:8476–8490. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Pang KC. Involvement of GABAergic and cholinergic medial septal neurons in hippocampal theta rhythm. Hippocampus. 2005;15:381–392. doi: 10.1002/hipo.20062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.