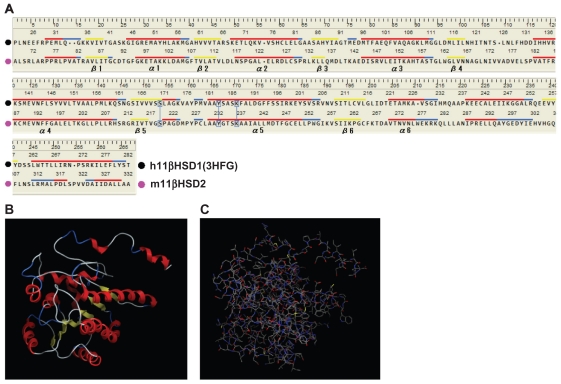
Figure 1.
Evaluation of the 2D and 3D structures of the m11βHSD2 model. (A) Homology-aligned sequences of h11βHSD1 (PDB code: 3HFG; black) and m11βHSD2 (magenta). Red line: α-helix. Blue line: turn. Yellow line: β-sheet. The alignments reveal that the cofactor-related GXXXGXG and catalytic YXXXK domains are conserved in m11βHSD2. The catalytic domain is often in the vicinity of a conserved S, and this is also the case for m11βHSD2. (B) The secondary structure of the m11βHSD2 model exhibits a central 6-stranded all-parallel β-sheet sandwich-like structure, flanked on both sides by 3-helices. (C) The constructed m11βHSD2 model. The model exhibits similar 3D structure to the structures of h11βHSD2 and 2.18,33