Abstract
The demonstrated ability of amphetamine to functionally activate the rat trace amine associated receptor 1 (rTAAR1) and the subsequent reports of amphetamine activation of TAAR1 in rhesus monkey mouse, human, and human-rat chimeric TAAR1-expressing cell lines has led to speculation as to the role of this receptor in the central nervous system (CNS) responses associated with amphetamine and its analogs. The agonist potencies of ten pairs of enantiomeric amphetamines, including several with known CNS activity, at primate TAAR1 stably expressed in RD-HGA16 cells, robustly indicate the S-configuration to be associated with higher potency. Moreover, the rank order of potency to activate TAAR1 parallels the stimulant action reported by humans for the specific amphetamines. Taken together, these data suggest that TAAR1 is a stereoselective binding site for amphetamine and that activation of TAAR1 is involved in the modulation of the stimulant properties of amphetamine and its congeners. In addition, the observed parallel between hTAAR1 and rhTAAR1 responses supports the rhesus monkey as a highly translational model for developing novel TAAR1-directed compounds as therapeutics for amphetamine-related addictions.
Keywords: Amphetamine, Stereoselective, TAAR1, Binding site, phenylisopropylamine
1. Introduction
Compounds in the amphetamine class have been known for decades to be highly addictive substances1 producing one or more of at least three distinct effects: an (S)- amphetamine-like effect, a DOM-like effect, and a PMMA-like effect.2 The DOM-like (hallucinogenic) effect has been associated with activation of the 5-HT2 family of receptors.3–5 In fact, the binding potencies of hallucinogenic amphetamines such as DOM, DOB, DOEt at 5HT2A/C receptors have been shown to correlate with measures of hallucinogenic potencies in rodent and the (R)–enantiomer has been found to possess higher potency than the (S)- enantiomer,6 consistent with binding data at 5-HT2A/C receptors. On the other hand, (S)- amphetamine, associated with stimulant properties, is bound at NET (EC50 = 7.1 nM) and DAT (EC50 = 24.8 nM) in cloned human cells7 and with low affinity at SERT (EC50 = 1.77 μM)7 and at rat adrenergic alpha sites (Ki~1 μM)8 but not at 5-HT receptors. Binding sites for (S)-[3H]amphetamine have been reported in rat brainstem, hypothalamus, and striatum.9 Further studies have characterized two sites for [3H]amphetamine: a low affinity sequestration-site lacking stereospecificity10 and a high affinity, stereoselective site of (S)-[3H]amphetamine incorporation in striatal synaptosomes.11 However, these studies did not implicate either of these two [3H]amphetamine sites with (S)-amphetamine-like stimulant effects. The demonstrated ability of amphetamine to functionally activate the rat trace amine associated receptor 1 (rTAAR1)12 and the subsequent reports of amphetamine activation of TAAR1 in rhesus monkey,13 rat,14 mouse,14–16 human,15, 17 and human-rat chimeric14 TAAR1-expressing cell lines has led to speculation as to the role of this receptor in the psychostimulant, hallucinogenic and addictive effects associated with amphetamine and its analogs18 as well as to the suggestion that TAAR1 may contribute a novel mode of action to these hallucinogenic drugs.19 A recent study of the activation of mouse, rat, and human-rat chimeric TAAR1s by amphetamine, methamphetamine, and p-hydroxyamphetamine14 concluded that this receptor could be a mediator of the effects of these drugs.
We had expressed wild type hTAAR1 in CHO cells stably expressing Gα1617, 20 and had developed a high throughput assay for functional TAAR1 agonists20 that we have been using to evaluate a series of amphetamines. Here, we have similarly expressed rhTAAR1 and have collected binding data on a spectrum of stereoisomers of amphetamines, including several with known CNS activity, that demonstrate that primate TAAR1 is a stereoselective binding site for compounds in the amphetamine class. The findings suggest an opportunity for the rhesus as a valid model for assessing whether specific TAAR1-active agents may have therapeutic efficacy in humans.
2. Methods
2.1 Cell Lines
2.1.1 Human TAAR1
A cell line expressing hTAAR1 was developed as previously described.20 Briefly, hTAAR1 cDNA was cloned from Marathon Ready cDNA from human stomach using the Advantage cDNA PCR kit (Clonetech, Mountain View, CA). The receptor coding sequence was amplified in 2 parts using primer pairs based on GenBank accession no. AF380185. The 2-part strategy was used because we had difficulty obtaining the full-length cDNA with 1 set of primers. The resultant PCR products were sub-cloned separately into the pcDNA4/HisMax TOPO TA expression vector (Invitrogen, Carlsbad, CA). The cloned PCR products were verified by sequence analysis (Duke University DNA Analysis Facility, Durham, NC). The full-length hTAAR1 cDNA was generated by restriction digestion of the 5′ and 3′ cDNAs and subsequent subcloning into the pcDNA4/HisMax TOPO TA expression vector. Upon reconsideration, we believed that the N-terminal 6× his tag in this vector might interfere with intracellular trafficking of the receptor by masking the native N-terminal. We therefore subcloned the coding region into an expression vector without an N-terminal tag. This expression vector contains an EF-1.2 promoter, a neomycin resistance gene, and a C-terminal HA epitope tag (pCEFL). The endogenous stop codon of hTAAR1 is included so the HA tag is not incorporated into the expressed hTAAR1 protein.
Plasmid DNA from the resulting construct was prepared using Qiagen’s Hispeed plasmid purification kit as per manufacturer’s instructions. The sequence was verified, and DNA was linearized by ScaI digestion prior to transfection of cells. The hTAAR1 expression construct was transfected into RD-HGA16 cells (Molecular Devices Corporation, Sunnyvale, CA). RD-HGA16 cells stably express the promiscuous Gq protein, Gα16. Expression of Gα16 allows coupling of hTAAR1 to calcium mobilization. These cells were transfected with the hTAAR1 expression construct using Lipofectamine 2000 reagent as per manufacturer’s instructions. Stably transfected cells were selected in 400 μg/ml geneticin (hTAAR1 selection) and 400 μg/ml hygromycin (Gα16 selection). Clones were selected from low-density cultures and analyzed for response to β-PEA using the Calcium 3 assay (Molecular Devices) and a FlexStation II384 (Molecular Devices) as per manufacturer’s instructions. Three positive clones were chosen for further experiments based on their positive response to β-PEA. All data are from 1 clonal cell line of hTAAR1/RD-HGA16 cells.
2.1.2 Rhesus TAAR1
A mammalian expression plasmid (pcDNA3.1 TOPO, Invitrogen) carrying an antibiotic resistance gene and the coding sequence for rhesus monkey TAAR113 was transiently transfected into RD-HGA16 cells using Lipofectamine Plus transfection reagent as per manufacturer’s instructions. The cells were transferred to 15 10-cm tissue culture dishes and subjected to antibiotic selection as described above for the hTAAR1 cells. Surviving clones were expanded and assayed for increased internal calcium concentration in response to treatment by a TAAR1 agonist using a calcium 4 kit and a FlexStation II384 fluorescence plate reader. For rhTAAR1, octopamine was the agonist of choice since it had been found that it was a superagonist relative to β-PEA (which we had used for hTAAR1).21
Screening of 400 clones that were obtained by transfection of the rhesus expression plasmid into RD-HGA16 cells, and that survived antibiotic selection over several transfection events, gave seven “hits.” However, none of these showed a reproducible response to 10 uM octopamine on subsequent analysis. Because our successful generation of stable cell lines expressing hTAAR1 had used the proprietary expression vector pCEFL, which was different from the vector containing the rhTAAR1, the rhTAAR1 coding sequence was subcloned into the pCEFL vector by restriction enzyme digestion, gel extraction, and ligation. Stable cell line generation using this expression plasmid was performed as above. Of 96 clones screened over one transfection event, ten “hits” were identified. Further analysis led to the identification of two cell lines stably expressing the rhTAAR1.
3. Results
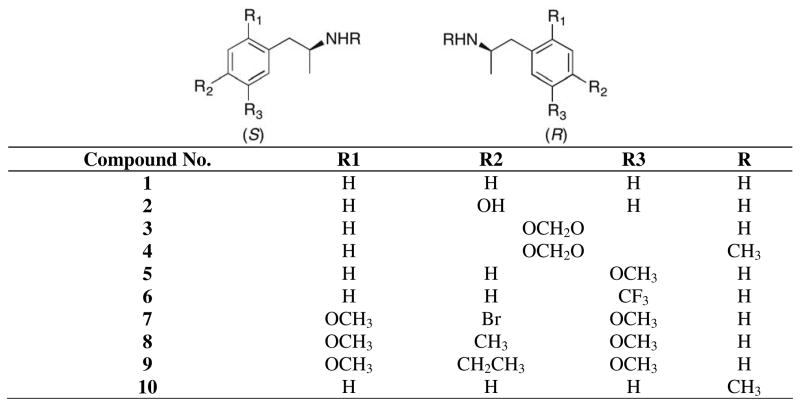
The potencies of ten pairs of enantiomeric amphetamines (1–10) to activate hTAAR117, 20 and rhTAAR1 stably expressed in RD-HGA16 cells, determined as previously described,20 are shown in Table 1. While all the data suggest possible stereoselectivity for the S-configuration (see Figure 1), the data for activation of hTAAR1 are not compelling for five (2, 3, 5, 8, 10) of the ten compound pairs. However, the potency data for activation of rhTAAR1 robustly indicate the S-configuration to be preferred for four of these five compounds (2, 3, 5, 8). The data for methamphetamine (10) are ambiguous.
Table 1.
Potencies to activate TAAR1
| No | Compound Name | EC50 (μM) | |
|---|---|---|---|
| Human | Rhesus | ||
| (S)-l | (S)-amphetamine | 0.6 ± 0.002 | 1.01 ± 0.08 |
| (R)-1 | (R)-amphetamine | 1.3 ± 0.03 | 1.86 ± 0.03 |
| (S)-2 | (S)-p-hydroxyamphetamine | 2.96 ± 0.1 | 0.101 ± 0.02 |
| (R)-2 | (R)-p-hydroxyamphetamine | 3.16 ± 1.2 | 0.424 ± 0.2 |
| (S)-3 | (S)-3,4-methylenedioxyamphetamine | 6.57 ± 1.2 | 1.35 ± 0.3 |
| (R)-3 | (R)-3,4-methylenedioxyamphetamine | 11.73 ± 6.3 | 2.48 ± 0.5 |
| (S)-4 | (S)-3,4-methylenedioxymethamphetamine | 73.7 ± 31 | 16.1 ± 6.8 |
| (R)-4 | (R)-3,4-methylenedioxymethamphetamine | inactive | 7.4 ± 0.5 |
| (S)-5 | (S)-m-methoxyamphetamine | 1.9 ± 0.9 | 3.3 ± 1.8 |
| (R)-5 | (R)-m-methoxyamphetamine | 6.5 ± 4.5 | inactive |
| (S)-6 | (S)-norfenfluramine | active* | ND |
| (R)-6 | (R)-norfenfluramine | inactive | ND |
| (S)-7 | (S)-4-bromo-2,5-dimethoxyamphetamine | 15.34 ± 0.1 | 2.14 ± 0.08 |
| (R)-7 | (R)-4-bromo-2,5-dimethoxyamphetamine | 31.94 ± 6.6 | 13.9 ± 5 |
| (S)-8 | (S)-4-methyl-2,5-dimethoxyamphetamine | inactive | 0.9 ± 0.04 |
| (R)-8 | (R)-4-methyl-2,5-dimethoxyamphetamine | inactive | inactive |
| (S)-9 | (S)-4-ethyl-2,5-dimethoxyamphetamine | 5.02 ± 1.8 | inactive |
| (R)-9 | (R)-4-ethyl-2,5-dimethoxyamphetamine | inactive | inactive |
| (S)-10 | (S)-methamphetamine | 1.5 ± 0.4 | 5.3 ± 0.5 |
| (R)-10 | (R)-methamphetamine | 3.3 ± 1.7 | 2.5 ± 1.2 |
ND= not determined
43% of maximum in screen, using 10 μM in hTAAR1/G 16 CHO-K1 cells loaded with Calcium 4 dye and analyzed using a FlexStation 2 microplate reader (Molecular Devices).
Figure 1.
Structures of Enantiomeric Amphetamines
4. Discussion
While a considerable body of quantitative data relative to potencies to activate m- and rTAAR1 has been reported,22–25 particularly for thyronamine and its analogs, no such data are reported for rhTAAR1, and only scant data are available for hTAAR1. For the few compounds where cross species comparisons have been possible, differences between human and rodent responses to structural variations have been observed. For example, addition of a p-hydroxyl functionality to the aromatic ring of β-PEA (to give tyramine) led to three-fold decreased potency for stimulation of cAMP formation in HEK 293 cells expressing hTAAR1 modified by replacing the N-terminal amino acids 1–20, the C-terminal amino acids 305–340, and the third intracellular loop corresponding to amino acids 204–258 with the corresponding rat TAAR1 sequences (h-rTAAR1), but resulted in five-fold increase in potency in rTAAR1 cells and in a two-fold reduction in mTAAR1 cells.15, 26 Wainscott et al. noted that addition of a p-hydroxyl functionality to the aromatic ring of β-PEA led to four-fold decreased potency for stimulation of cAMP formation in hTAAR1-expressing rGasAV12-664 cells, while potency for stimulation of cAMP formation in mTAAR1-expressing rGasAV12-664 cells was increased by an order of magnitude;15 analogous results were reported for EC50 values for cAMP accumulation in HEK cells stably expressing rTAAR1 and the chimeric h-rTAAR1.14 Similarly, while the potencies for β-PEA in human and mouse TAAR1 were almost the same (EC50 = 106 nM and 209 nM, respectively), they were two orders of magnitude different for 3-iodothyronamine (EC50 = 1510 nM and 22.4 nM, for human and mouse, respectively). Addition of a p-hydroxy functionality to amphetamine virtually abolished activity at h-rTAAR1 but had minimal effect in rTAAR1 and a stereoselective effect in mTAAR1.14 These observations may be due to, as has been pointed out,13 the significant divergence in sequence between human and rodent TAAR1s (76–78%).27 Since the rhTAAR1 coding sequence had been found to be 96.9% homologous to the hTAAR1 coding sequence,28 we undertook to develop a stable expression of rhTAAR1 in cells stably expressing Gα16, by analogy to our previously developed expression system for hTAAR117, 20, 29 for the purpose of making direct comparisons between hTAAR1 and rhTAAR1.
Only scant data relative to the potencies of amphetamine and its analogs to activate TAAR1 are currently available (Table 2). The available data are in reasonable agreement considering that the evaluations have been carried out in different laboratories and using different expression systems and bioassay methodologies. A significant exception are the data reporting (S)-amphetamine, with EC50 = 2 nM, to be the most potent agonist identified for mTAAR1.16 Our results (Table 1) for amphetamine (1) are similar to the literature data. Specifically, the EC50 (0.6 ± 0.002 μM) obtained by us for (S)-amphetamine ((S)-1) to stimulate calcium flux by activation of hTAAR1 transfected into RD-HGA16 cells is in between the EC50 values obtained by evaluating the effectiveness of (S)-1 to promote cAMP accumulation in hTAAR1-expressing rGasAV12-664 cells (0.99 ± 0.16 μM),15 and by measuring the cAMP signaling associated with activation of hTAAR1 modified by the addition of a glycosylation site by insertion of the first nine amino acids of the human β2-andrenergic receptor between the HA-tag and the N-terminus (0.14 ± 0.01 μM).30 Similarly, for (R)-amphetamine ((R)-1) our EC50 value of 1.3 ± 0.3 μM, is in the range of 1.7 ± 0.29 μM and 0.25 ± 0.01 μM reported by Wainscott15 and Barak,30 respectively. Our data diverge significantly from those of Barak et al.30 for (S)-3,4 methylenedioxymethamphetamine ((S)-4). In fact, the potency (0.37 ± 0.05 μM) reported by Barak et al.30 for (S)-4, is surprisingly high, particularly when considering that effects on potency reported by Barak et al. parallel our previously reported findings for analogs of β-PEA.17 For example, the EC50 reported for (S)- methamphetamine (1.3 ± 0.19 μM) is in good agreement with the value determined by us (1.5 ± 0.4 μM), suggesting that N-methylation tends to decrease potency at hTAAR1, in agreement with our finding that N-methylation of β-PEA decreased potency at hTAAR1 by a factor of three.17 Since we had found that 3,4- methylenedioxy substitution onto the aromatic moiety of β-PEA decreased potency at hTAAR1 by at least an order of magnitude, an EC50 value of 30 μM might be expected for (S)-MDMA ((S)-4) in reasonable agreement with the value obtained by us (Table 1) but much higher than the value reported by Barak et al.30 Overall, introduction of oxygenated substituents at the aryl moiety of amphetamine (see 2, 3, 4, 5, 7, 8, 9) decreased potency at hTAAR1, as had been noted for analogous substitution in β-PEA.17
Table 2.
Literature Data for Potencies to Activate TAAR1
| Compound | EC50 (μM) | |||
|---|---|---|---|---|
| Rat | Mouse | Human-rat | Human | |
| (S)-1 | 0.8114 | 0.2114 | 1.1214 | 0.99 ± 0.1615 |
| 0.44 ± 0.0112 | 0.002 ± 0.00116 | 0.14 ± 0.0130 | ||
| 1.2 ± 0.0715 | ||||
| (R)-1 | 0.2814 | 4.9614 | 3.0914 | 1.7 ± 0.2915 |
| 0.21 ± 0.0412 | 0.065 ± 0.05316 | 0.25 ± 0.0130 | ||
| 1.4 ± 0.615 | ||||
| (rac)-2 | 0.05 ± 0.0112 | |||
| (S)-2 | 0.1914 | 0.2814 | >5.4214 | |
| (R)-2 | 0.0614 | 5.6514 | >8.5412,14 | |
| (rac)-4 | 1.7 ± 1.212 | |||
| (S)-4 | 0.37 ± 0.0530 | |||
| (S)-10 | 0.8914 | 0.9214 | 4.4414 | 1.3 ± 0.1930 |
| 0.07 ± 0.0816 | ||||
| (R)-10 | 1.1914 | 2.4414 | 9.8314 | |
Based on studies carried out in rat brain synaptosomal preparations it has been suggested that the discrete behavioral effects of high and low doses of (S)-amphetamine ((S)-1) might be attributable to the low affinity amphetamine sequestration site and the high affinity amphetamine transport site, respectively.10, 11 Specifically, low doses of (S)-1 would be bound by the high affinity amphetamine transport site thereby leading to increased monoamine release. Studies of the effects of ephedrine-related structures on biogenic amine uptake and release in stably and transiently transfected cells expressing mainly human cloned transporters demonstrated high activity for (S)-1 at norepinephrine (EC50 = 7.1 nM) and dopamine (EC50 = 24.8 nM) transporters,7 and it was suggested that DA release by (S)-1 may contribute to its behavioral effects. Co-localization of TAAR1with DAT in some, but not all, dopamine neurons has been reported, and it has been shown that activation of TAAR1 plays a mediatory role in DAT regulation.31, 32 In particular, it has been shown that β-PEA and methamphetamine effects in cells expressing TAAR-DAT significantly exceed those observed in cells expressing DAT only. Consistent with this conclusion is the higher potency of (S)-1 in rat synaptosomes relative to cloned human DAT cells (EC50 60 nM vs 240 nM). These data suggest that the efficacy of different TAAR1 agonists at DAT would be related to their affinity for TAAR1 and their efficiency as substrates at the DAT. It is thus possible that the stimulant properties of (S)- amphetamine ((S)-1), and of analogs that generalize to (S)-1, may be regulated by TAAR1. Some of the similarities between behaviors elicited by β-PEA and (S)-1, which were observed decades ago, may also be accounted for by binding at TAAR1. For example, β-PEA was found to be as effective as amphetamine in a place preference paradigm in rats although it was significantly less potent than either amphetamine isomer.33
It has been long known that the addition of substituents to the aromatic moiety of amphetamine results in cognitive and behavioral outcomes distinct from those associated with (S)-amphetamine. Thus, an early study described (S)-1 as a central stimulant with anorectic properties and effects on cardiovascular and thermoregulatory processes.34 The same report described the effects of aryl substituted phenylisopropylamines as retaining amphetamine-like effects while exhibiting LSD-like activity.34 Considering what relevance the in vitro potencies of the series of psychoactive amphetamines to activate primate TAAR1 may have to the in vivo effects of these compounds in primates is clearly premature. First, because the distribution parameters in primates are not known and, second, no validated studies in primates are available. Keeping these caveats in mind, it is nevertheless interesting to note that our results are consistent with the possibility that activation of TAAR1 contributes to amphetamine-like effects. In particular, our data show (S)-3 to be twice as potent as (R)-3 at both rhTAAR1 and hTAAR1, and (R)-4 to be inactive at hTAAR1 while (S)-4 retained weak activity, consistent with the observation that for MDA (3) and MDMA (4) the (S)- isomers, but not the (R)-isomers, substitute for (S)-amphetamine in drug discrimination studies in rats2, 35 and with the notion that psychomotor stimulant activity is associated with the (S)-configuration of these agents.36 In humans doses of 100 mg (or greater) of (S)-3 or (S)-4, but not of (R)-3 or (R)-4, have been reported to lead to excitation.37 Similar effects have been reported for 2.6 mg doses of (S) –DOM ((S)-8), but not for the enantiomeric (R)-8;37 our data show (S)-8 to be equipotent to (S)-1 in rhTAAR1, consistent with the lower effective in vivo dose of (S)-8 relative to (S)-3 or (S)-4.37 The lack of observed agonist activity for (S)-8 in hTAAR1 is surprising. Our data show (S)-m-methoxyamphetamine ((S)-5) to have relatively high potency at both rhTAAR1 and hTAAR1, suggesting that (S)-5 would have significant amphetamine-like effects. Drug discrimination studies in rats showed racemic m-methoxyamphetamine (5) to produce amphetamine-like effect and to generalize to (S)-138 but not to DOM (8); examination of the individual enantiomers of 5 in drug discrimination studies showed significantly reduced response rates and disruption of behavior by both enantiomers.39 Relatively high potency at hTAAR1 has also been found for (S)- 2,5-dimethoxy-4-ethylamphetamine ((S)-9), again suggesting that it may produce amphetaminelike effects. The literature data for racemic (S)-9 imply some anxiogenic effects in humans at low dose (25 mg);37 but no clear-cut amphetamine-like stimuli of either of the individual enantiomers or of the racemate were detected in a study in which human subjects were given oral doses of 1–4 mg of 9.40 The bromo analog (S)-7 has been reported to have no effect in humans at doses of 0.5–1.0 mg. If, indeed, psychostimulant effects are associated with activation of TAAR1 this observation may be consistent with the low agonist potency observed for (S)-7 in hTAAR1. There are no literature reports concerning the effects of (S)-7 in rhesus monkeys. While we did not collect quantitative data on N-norfenfluramine (6), the fact that only (S)-6 was found to be active at hTAAR1 is consistent with the finding that (S)-6 was more potent than (R)-6 in counteracting amphetamine induced increase in locomotor activity in rats.41 While this effect is likely to be associated with activation of 5-HT2 activation, it does not preclude involvement of TAAR1.
While our data suggest a role for TAAR1 in eliciting amphetamine-like stimulant effects, it must be borne in mind that the observed in vivo effects are likely to result from interaction with both TAAR1 and monoamine transporters. Thus it has been shown that the selective TAAR1 agonist RO5166017 fully prevented psychostimulant-induced and persistent hyperdopaminergia-related hyperactivity in mice.42 This effect was found to be DAT-independent, since suppression of hyperactivity was observed in DAT-KO mice. 42
The collected information leads us to conclude that TAAR1 is a stereoselective binding site for amphetamine and that TAAR1 activation by amphetamine and its congeners may contribute to the stimulant properties of this class of compounds. Since the observed S-stereoselectivity for activation of TAAR1 is inconsistent with the known R-stereoselectivity for hallucinogenic activity of compounds in the amphetamine class, we conclude that TAAR1 is not a mediator of hallucinogenic activity of these agents. Our results show a reasonable parallel between hTAAR1 and rhTAAR1 responses to a series of substituted amphetamines, supporting the rhesus monkey as a highly translational model for developing novel TAAR1-directed compounds as therapeutics for amphetamine-related addictions.
Acknowledgments
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant: RO1-DA125] (A.H.L.); National Institutes of Health National Institute on Drug Abuse [Grant: DA025802] (G.M.M. K02 salary support); The National Institutes of Health, National Center for Research Resources, New England Primate Research Center [Grant: RR00168] (G.M.M. base grant of NEPRC from NCRR); and National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant: 1R21NS064780-01A1] (B.G.). With special thanks to Dr. Ramón Trullas, Institut d’Investigacions Biomediques de Barcelona, Barcelona, Spain) for his kind gift of pCEFL.
Abbreviations
- TAAR
trace amine-associated receptor
- NET
norepinephrine transporter
- DAT
dopamine transporter
- SERT
serotonin transporter
- 5-HT
5-hydroxytryptamine
- LSD
lysergic acid diethylamide
- CHO
Chinese hamster ovary
- DOM
2,5-dimethoxy-4-methylamphetamine
- PMMA
p-methoxymethamphetamine
- MDA
3,4-methylenedioxyamphetamine
- MDMA
3,4-methylenedioxymethamphetamine
- CNS
central nervous system
- DOB
4-Bromo-2,5-dimethoxyamphetamine
- DOEt
2,5-Dimethoxy-4-ethylamphetamine
- KO
knock out
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shulgin AT, Sargent T, Naranjo C. Nature. 1969;221:537. doi: 10.1038/221537a0. [DOI] [PubMed] [Google Scholar]
- 2.Glennon RA, Young R. Pharm Biochem Behav. 2002;72:307. doi: 10.1016/s0091-3057(01)00770-5. [DOI] [PubMed] [Google Scholar]
- 3.Branchek T, Adham N, Macchi M, Kao HT, Hartig PR. Mol Pharmacol. 1990;38:604. [PubMed] [Google Scholar]
- 4.Leonhardt S, Titeler M. J Neurochem. 1989;53:316. doi: 10.1111/j.1471-4159.1989.tb07334.x. [DOI] [PubMed] [Google Scholar]
- 5.Pierce PA, Peroutka SJ. J Neurochem. 1989;52:656. doi: 10.1111/j.1471-4159.1989.tb09171.x. [DOI] [PubMed] [Google Scholar]
- 6.McLean TH, Parrish JC, Braden MR, Marona-Lewicka D, Gallardo-Godoy A, Nichols DE. J Med Chem. 2006;49:5794. doi: 10.1021/jm060656o. [DOI] [PubMed] [Google Scholar]
- 7.Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, Birkes J, Young R, Glennon RA. J Pharmacol Exp Ther. 2003;307:138. doi: 10.1124/jpet.103.053975. [DOI] [PubMed] [Google Scholar]
- 8.U’Prichard DC, Greenberg DA, Snyder SH. Mol Pharmacol. 1977;13:454. [PubMed] [Google Scholar]
- 9.Hauger RL, Hulihan-Giblin B, Skolnick P, MPS Life Sci. 1984;34:771. doi: 10.1016/0024-3205(84)90385-0. [DOI] [PubMed] [Google Scholar]
- 10.Zaczek R, Culp S, Goldberg H, McCann DJ, De Souza EB. J Pharmacol Exp Ther. 1991;257:820. [PubMed] [Google Scholar]
- 11.Zaczek R, Culp S, De Souza EB. J Pharmacol Exp Ther. 1991;257:830. [PubMed] [Google Scholar]
- 12.Bunzow JR, Sonders MS, Arttmagangkul S, Harrison LM, Zhamg G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, Olson SB, Magenis RE, Amara SG, Grandy DK. Mol Pharmacol. 2001;60:1181. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- 13.Miller GM, Verrico C, Jassen A, Konar M, Yang H, Panas H, Mary B, Johnson R, Madras BK. J Pharmacol Exp Ther. 2005;313:983. doi: 10.1124/jpet.105.084459. [DOI] [PubMed] [Google Scholar]
- 14.Reese EA, Bunzow JR, Arttamangkul S, Sonders MS, Grandy DK. J Pharmacol Exp Ther. 2007;321:178. doi: 10.1124/jpet.106.115402. [DOI] [PubMed] [Google Scholar]
- 15.Wainscott DB, Little SP, Yin T, Tu Y, Rocco VP, He JX, Nelson DL. J Pharmacol Exp Ther. 2007;320:475. doi: 10.1124/jpet.106.112532. [DOI] [PubMed] [Google Scholar]
- 16.Wolinsky TD, Swanson CJ, Smith KE, Zhong H, Borowsky S, Seeman P, Branhek T, Gerald CP. Genes Brain Behav. 2007;6:628. doi: 10.1111/j.1601-183X.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 17.Lewin AH, Navarro HA, Mascarella SW. Bioorg Med Chem. 2008;16:7415. doi: 10.1016/j.bmc.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zucchi R, Chiellini G, Scanlan TS, Grandy DK. Br J Pharmacol. 2006;149:967. doi: 10.1038/sj.bjp.0706948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burchett SA, Hicks TP. Prog Neurobiol. 2006;79:223. doi: 10.1016/j.pneurobio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Navarro HA, Gilmour BP, Lewin AH. J Biomol Screen. 2006;11:688. doi: 10.1177/1087057106289891. [DOI] [PubMed] [Google Scholar]
- 21.Xie Z, Miller GM. J Pharmacol Exp Ther. 2007;321:128. doi: 10.1124/jpet.106.117382. [DOI] [PubMed] [Google Scholar]
- 22.Hart ME, Suchland KL, Miyakawa M, Bunzow JR, Grandy DK, Scanlan TS. J Med Chem. 2006;49:1101. doi: 10.1021/jm0505718. [DOI] [PubMed] [Google Scholar]
- 23.Scanlan TS, Suchland KL, Hart ME, Chiellini G, Huang Y, Kruzich PJ, Frascarelli S, Crossley DA, Bunzow JR, Ronca-Testoni S, Lin ET, Hatton D, Zucchi R, Grandy DK. Nat Med. 2004;10:638. doi: 10.1038/nm1051. [DOI] [PubMed] [Google Scholar]
- 24.Tan ES, Miyakawa M, Bunzow JR, Grandy DK, Scanlan TS. J Med Chem. 2007;50:2787. doi: 10.1021/jm0700417. [DOI] [PubMed] [Google Scholar]
- 25.Tan ES, Naylor JC, Groban ES, Bunzow JR, Jacobson MP, Grandy DK, Scanlan TS. ACS Chem Biol. 2009;4:209. doi: 10.1021/cb800304d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindemann L, Ebeling M, Kratochwil NA, Bunzow JR, Grandy DK, Hoener MC. Genomics. 2005;85:372. doi: 10.1016/j.ygeno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Borowsky B, Adham N, Jones KA, Raddatz R, Arymshym R, Ogozalek KL, Durkin MM, Laklani PP, Bonini JA, Pathirana S, Boyle N, Po X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C. Proc Nat’l Acad Sci USA. 2001;98:8966. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller GM, Madras BK. Poster Presentation: A trace amine receptor (TAR1) is a novel amphetamine receptor in primate brain; Bal Harbour, FL. 2003. [Google Scholar]
- 29.Lewin AH. In: Drug Addiction from Basic Research to Therapy. Rapaka RS, Sadee W, editors. Springer; New York: 2008. p. 327. [Google Scholar]
- 30.Barak LS, Salahpour A, Zhang X, Masri B, Sotnikova TD, Ramsey AJ, Violin JD, Lefkowitz RJ, Caron MG, Gainetdinov RR. Mol Pharmacol. 2008;74:585. doi: 10.1124/mol.108.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Z, Miller GM. Biochem Pharmacol. 2009;78:1095. doi: 10.1016/j.bcp.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Z, Miller GM. J Pharmacol Exp Ther. 2009;330:316. doi: 10.1124/jpet.109.153775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilbert D, Cooper SJ. Eur J Pharmacol. 1983;95:311. doi: 10.1016/0014-2999(83)90653-2. [DOI] [PubMed] [Google Scholar]
- 34.Aldous FAB, Barrass BC, Brewster K, Buxton DA, Green DM, Pinder RM, Rich P, Skeels M, Tutt KJ. J Med Chem. 1974;17:1100. doi: 10.1021/jm00256a016. [DOI] [PubMed] [Google Scholar]
- 35.Glennon RA, Young R, Dukat M, Cheng Y. Pharmacol Biochem Behav. 1997;57:151. doi: 10.1016/s0091-3057(96)00306-1. [DOI] [PubMed] [Google Scholar]
- 36.Murnane KS, Fantegrossi WE, Godfrey JR, Banks ML, Howell LL. J Pharmacol Exp Ther. 2010;334:642. doi: 10.1124/jpet.110.166595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shulgin A, Shulgin A. PIHKAL: Phenethylamines I Have Known And Loved: A Chemical Love Story. 2010 online version: http://www.erowid.org/library/books_online/pihkal/pihkal.shtml.
- 38.Glennon RA, Young R, Hauck AE. Pharmacol Biochem Behav. 1985;22:723. doi: 10.1016/0091-3057(85)90520-9. [DOI] [PubMed] [Google Scholar]
- 39.Glennon RA, Young R, Dukat M, Chang-Fong J, El-Zahabi M. Pharmacol Biochem Behav. 2007;86:477. doi: 10.1016/j.pbb.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snyder SH, Unger S, Blatchley R, Barfknecht CF. Arch Gen Psychiatry. 1974;31:103. doi: 10.1001/archpsyc.1974.01760130079013. [DOI] [PubMed] [Google Scholar]
- 41.Bendotti C, Borsini F, Zanini MG, Samanin R, Garattini S. Pharmacol Res Commun. 1980;12:567. doi: 10.1016/s0031-6989(80)80142-1. [DOI] [PubMed] [Google Scholar]
- 42.Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, Durkin S, Zbinden KG, Norcross R, Meyer CA, Metzler V, Chaboz S, Ozmen L, Trube G, Pouzet B, Bettler B, Caron MG, Wettstein JG, Hoener MC. Proc Natl Acad Sci U S A. 2011;108:8485. doi: 10.1073/pnas.1103029108. [DOI] [PMC free article] [PubMed] [Google Scholar]