Abstract
The etiologic agent of the acquired immune deficiency syndrome, human T-cell lymphotropic virus type III (HTLV-III), has recently been shown to morphologically resemble and share sequence homology with visna virus, a pathogenic lentivirus. Molecular hybridization, heteroduplex mapping, and DNA sequence analyses were used to compare HTLV-III to other lentiviruses of domestic animals, including visna, caprine arthritis encephalitis, and equine infectious anemia viruses. Hybridization results showed that a substantial amount of sequence homology exists between each of these viruses and HTLV-III. In addition, a closer relationship was found between visna and caprine arthritis encephalitis viruses than for any of the other lentiviruses studied. These results, along with nucleotide and amino acid sequence comparisons, have been used in a comprehensive effort to derive a systematic relationship for lentiviruses and to provide further evidence for classifying HTLV-III with the Lentivirinae subfamily of retroviruses. This relationship predicts that similarities in biology and disease process can be expected between HTLV-III and other Lentivirinae members.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. S., Crawford T. B., Klevjer-Anderson P. A pathogenetic study of the early connective tissue lesions of viral caprine arthritis-encephalitis. Am J Pathol. 1980 May;99(2):257–278. [PMC free article] [PubMed] [Google Scholar]
- Alizon M., Sonigo P., Barré-Sinoussi F., Chermann J. C., Tiollais P., Montagnier L., Wain-Hobson S. Molecular cloning of lymphadenopathy-associated virus. Nature. 1984 Dec 20;312(5996):757–760. doi: 10.1038/312757a0. [DOI] [PubMed] [Google Scholar]
- Casey J. M., Kim Y., Andersen P. R., Watson K. F., Fox J. L., Devare S. G. Human T-cell lymphotropic virus type III: immunologic characterization and primary structure analysis of the major internal protein, p24. J Virol. 1985 Aug;55(2):417–423. doi: 10.1128/jvi.55.2.417-423.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu I. M., Callahan R., Tronick S. R., Schlom J., Aaronson S. A. Major pol gene progenitors in the evolution of oncoviruses. Science. 1984 Jan 27;223(4634):364–370. doi: 10.1126/science.6197754. [DOI] [PubMed] [Google Scholar]
- Chiu I. M., Yaniv A., Dahlberg J. E., Gazit A., Skuntz S. F., Tronick S. R., Aaronson S. A. Nucleotide sequence evidence for relationship of AIDS retrovirus to lentiviruses. 1985 Sep 26-Oct 2Nature. 317(6035):366–368. doi: 10.1038/317366a0. [DOI] [PubMed] [Google Scholar]
- Cork L. C., Hadlow W. J., Crawford T. B., Gorham J. R., Piper R. C. Infectious leukoencephalomyelitis of young goats. J Infect Dis. 1974 Feb;129(2):134–141. doi: 10.1093/infdis/129.2.134. [DOI] [PubMed] [Google Scholar]
- Cork L. C., Narayan O. The pathogenesis of viral leukoencephalomyelitis-arthritis of goats. I. Persistent viral infection with progressive pathologic changes. Lab Invest. 1980 Jun;42(6):596–602. [PubMed] [Google Scholar]
- Coward J. E., Harter D. H., Morgan C. Electron microscopic observations of Visna virus-infected cell cultures. Virology. 1970 Apr;40(4):1030–1038. doi: 10.1016/0042-6822(70)90149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford T. B., Adams D. S., Cheevers W. P., Cork L. C. Chronic arthritis in goats caused by a retrovirus. Science. 1980 Feb 29;207(4434):997–999. doi: 10.1126/science.6153243. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Derse D., Caradonna S. J., Casey J. W. Bovine leukemia virus long terminal repeat: a cell type-specific promoter. Science. 1985 Jan 18;227(4684):317–320. doi: 10.1126/science.2981431. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Gonda M. A., Charman H. P., Walker J. L., Coggins L. Scanning and transmission electron microscopic study of equine infectious anemia virus. Am J Vet Res. 1978 May;39(5):731–740. [PubMed] [Google Scholar]
- Gonda M. A., Wong-Staal F., Gallo R. C., Clements J. E., Narayan O., Gilden R. V. Sequence homology and morphologic similarity of HTLV-III and visna virus, a pathogenic lentivirus. Science. 1985 Jan 11;227(4683):173–177. doi: 10.1126/science.2981428. [DOI] [PubMed] [Google Scholar]
- Haase A. T. The slow infection caused by visna virus. Curr Top Microbiol Immunol. 1975;72:101–156. doi: 10.1007/978-3-642-66289-8_4. [DOI] [PubMed] [Google Scholar]
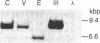
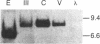
- Hahn B. H., Gonda M. A., Shaw G. M., Popovic M., Hoxie J. A., Gallo R. C., Wong-Staal F. Genomic diversity of the acquired immune deficiency syndrome virus HTLV-III: different viruses exhibit greatest divergence in their envelope genes. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4813–4817. doi: 10.1073/pnas.82.14.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Arya S. K., Popovic M., Gallo R. C., Wong-Staal F. Molecular cloning and characterization of the HTLV-III virus associated with AIDS. Nature. 1984 Nov 8;312(5990):166–169. doi: 10.1038/312166a0. [DOI] [PubMed] [Google Scholar]
- Hess J. L., Clements J. E., Narayan O. cis- and trans-acting transcriptional regulation of visna virus. Science. 1985 Aug 2;229(4712):482–485. doi: 10.1126/science.2990051. [DOI] [PubMed] [Google Scholar]
- Kanki P. J., McLane M. F., King N. W., Jr, Letvin N. L., Hunt R. D., Sehgal P., Daniel M. D., Desrosiers R. C., Essex M. Serologic identification and characterization of a macaque T-lymphotropic retrovirus closely related to HTLV-III. Science. 1985 Jun 7;228(4704):1199–1201. doi: 10.1126/science.3873705. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Kono Y. Propagation and titration of equine infectious anemia virus in horse leukocyte culture. Natl Inst Anim Health Q (Tokyo) 1967 Spring;7(1):8–20. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- Molineaux S., Clements J. E. Molecular cloning of unintegrated visna viral DNA and characterization of frequent deletions in the 3' terminus. Gene. 1983 Aug;23(2):137–148. doi: 10.1016/0378-1119(83)90045-8. [DOI] [PubMed] [Google Scholar]
- Mullins J. I., Casey J. W., Nicolson M. O., Davidson N. Sequence organization of feline leukemia virus DNA in infected cells. Nucleic Acids Res. 1980 Aug 11;8(15):3287–3305. doi: 10.1093/nar/8.15.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Chase J. Antigenic shift of visna virus in persistently infected sheep. Science. 1977 Jul 22;197(4301):376–378. doi: 10.1126/science.195339. [DOI] [PubMed] [Google Scholar]
- Narayan O., Kennedy-Stoskopf S., Sheffer D., Griffin D. E., Clements J. E. Activation of caprine arthritis-encephalitis virus expression during maturation of monocytes to macrophages. Infect Immun. 1983 Jul;41(1):67–73. doi: 10.1128/iai.41.1.67-73.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Sheffer D., Griffin D. E., Clements J., Hess J. Lack of neutralizing antibodies to caprine arthritis-encephalitis lentivirus in persistently infected goats can be overcome by immunization with inactivated Mycobacterium tuberculosis. J Virol. 1984 Feb;49(2):349–355. doi: 10.1128/jvi.49.2.349-355.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Wolinsky J. S., Clements J. E., Strandberg J. D., Griffin D. E., Cork L. C. Slow virus replication: the role of macrophages in the persistence and expression of visna viruses of sheep and goats. J Gen Virol. 1982 Apr;59(Pt 2):345–356. doi: 10.1099/0022-1317-59-2-345. [DOI] [PubMed] [Google Scholar]
- Payne S., Parekh B., Montelaro R. C., Issel C. J. Genomic alterations associated with persistent infections by equine infectious anaemia virus, a retrovirus. J Gen Virol. 1984 Aug;65(Pt 8):1395–1399. doi: 10.1099/0022-1317-65-8-1395. [DOI] [PubMed] [Google Scholar]
- Payne S., Parekh B., Montelaro R. C., Issel C. J. Genomic alterations associated with persistent infections by equine infectious anaemia virus, a retrovirus. J Gen Virol. 1984 Aug;65(Pt 8):1395–1399. doi: 10.1099/0022-1317-65-8-1395. [DOI] [PubMed] [Google Scholar]
- Pyper J. M., Clements J. E., Molineaux S. M., Narayan O. Genetic variation among lentiviruses: homology between visna virus and caprine arthritis-encephalitis virus is confined to the 5' gag-pol region and a small portion of the env gene. J Virol. 1984 Sep;51(3):713–721. doi: 10.1128/jvi.51.3.713-721.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice N. R., Stephens R. M., Burny A., Gilden R. V. The gag and pol genes of bovine leukemia virus: nucleotide sequence and analysis. Virology. 1985 Apr 30;142(2):357–377. doi: 10.1016/0042-6822(85)90344-7. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Stephens R. M., Couez D., Deschamps J., Kettmann R., Burny A., Gilden R. V. The nucleotide sequence of the env gene and post-env region of bovine leukemia virus. Virology. 1984 Oct 15;138(1):82–93. doi: 10.1016/0042-6822(84)90149-1. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robert-Guroff M., Brown M., Gallo R. C. HTLV-III-neutralizing antibodies in patients with AIDS and AIDS-related complex. Nature. 1985 Jul 4;316(6023):72–74. doi: 10.1038/316072a0. [DOI] [PubMed] [Google Scholar]
- Robey W. G., Safai B., Oroszlan S., Arthur L. O., Gonda M. A., Gallo R. C., Fischinger P. J. Characterization of envelope and core structural gene products of HTLV-III with sera from AIDS patients. Science. 1985 May 3;228(4699):593–595. doi: 10.1126/science.2984774. [DOI] [PubMed] [Google Scholar]
- SIGURDSSON B., PALSSON P., GRIMSSON H. Visna, a demyelinating transmissible disease of sheep. J Neuropathol Exp Neurol. 1957 Jul;16(3):389–403. doi: 10.1097/00005072-195707000-00010. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Power M. D., Barr P. J., Steimer K. S., Stempien M. M., Brown-Shimer S. L., Gee W. W., Renard A., Randolph A., Levy J. A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science. 1985 Feb 1;227(4686):484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G. M., Harper M. E., Hahn B. H., Epstein L. G., Gajdusek D. C., Price R. W., Navia B. A., Petito C. K., O'Hara C. J., Groopman J. E. HTLV-III infection in brains of children and adults with AIDS encephalopathy. Science. 1985 Jan 11;227(4683):177–182. doi: 10.1126/science.2981429. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Patarca R., Rosen C., Wong-Staal F., Haseltine W. Location of the trans-activating region on the genome of human T-cell lymphotropic virus type III. Science. 1985 Jul 5;229(4708):74–77. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Rosen C., Goh W. C., Haseltine W. A transcriptional activator protein encoded by the x-lor region of the human T-cell leukemia virus. Science. 1985 Jun 21;228(4706):1430–1434. doi: 10.1126/science.2990028. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Rosen C., Wong-Staal F., Salahuddin S. Z., Popovic M., Arya S., Gallo R. C., Haseltine W. A. Trans-acting transcriptional regulation of human T-cell leukemia virus type III long terminal repeat. Science. 1985 Jan 11;227(4683):171–173. doi: 10.1126/science.2981427. [DOI] [PubMed] [Google Scholar]
- Sonigo P., Alizon M., Staskus K., Klatzmann D., Cole S., Danos O., Retzel E., Tiollais P., Haase A., Wain-Hobson S. Nucleotide sequence of the visna lentivirus: relationship to the AIDS virus. Cell. 1985 Aug;42(1):369–382. doi: 10.1016/s0092-8674(85)80132-x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stephens R. M., Casey J. W., Rice N. R. Equine infectious anemia virus gag and pol genes: relatedness to visna and AIDS virus. Science. 1986 Feb 7;231(4738):589–594. doi: 10.1126/science.3003905. [DOI] [PubMed] [Google Scholar]
- THORMAR H. An electron microscope study of tissue cultures infected with visna virus. Virology. 1961 Aug;14:463–475. doi: 10.1016/0042-6822(61)90339-7. [DOI] [PubMed] [Google Scholar]
- Van der Maaten M. J., Boothe A. D., Seger C. L. Isolation of a virus from cattle with persistent lymphocytosis. J Natl Cancer Inst. 1972 Dec;49(6):1649–1657. doi: 10.1093/jnci/49.6.1649. [DOI] [PubMed] [Google Scholar]
- Veronese F. D., DeVico A. L., Copeland T. D., Oroszlan S., Gallo R. C., Sarngadharan M. G. Characterization of gp41 as the transmembrane protein coded by the HTLV-III/LAV envelope gene. Science. 1985 Sep 27;229(4720):1402–1405. doi: 10.1126/science.2994223. [DOI] [PubMed] [Google Scholar]
- Vigne R., Filippi P., Quérat G., Sauze N., Vitu C., Russo P., Delori P. Precursor polypeptides to structural proteins of visna virus. J Virol. 1982 Jun;42(3):1046–1056. doi: 10.1128/jvi.42.3.1046-1056.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain-Hobson S., Alizon M., Montagnier L. Relationship of AIDS to other retroviruses. 1985 Feb 28-Mar 6Nature. 313(6005):743–743. doi: 10.1038/313743a0. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Clapham P. R., Cheingsong-Popov R., Dalgleish A. G., Carne C. A., Weller I. V., Tedder R. S. Neutralization of human T-lymphotropic virus type III by sera of AIDS and AIDS-risk patients. Nature. 1985 Jul 4;316(6023):69–72. doi: 10.1038/316069a0. [DOI] [PubMed] [Google Scholar]
- Wong-Staal F., Gallo R. C. Human T-lymphotropic retroviruses. Nature. 1985 Oct 3;317(6036):395–403. doi: 10.1038/317395a0. [DOI] [PubMed] [Google Scholar]
- Wong-Staal F., Shaw G. M., Hahn B. H., Salahuddin S. Z., Popovic M., Markham P., Redfield R., Gallo R. C. Genomic diversity of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Aug 23;229(4715):759–762. doi: 10.1126/science.2992084. [DOI] [PubMed] [Google Scholar]