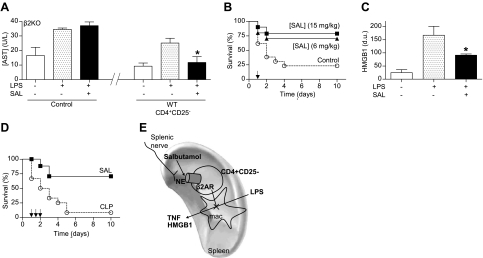
Figure 5.
β2-Agonist prevents organ damage and rescues mice from established polymicrobial peritonitis. A) Adult β2-knockout (β2KO) mice were given vehicle (control), or CD3+CD4+CD25− lymphocytes from wild-type (WT) mice with vehicle or β2-agonist salbutamol (SAL; 15 mg/kg i.p.). Serum aspartate transaminase (AST) levels were analyzed at 90 min after LPS. *P < 0.01 vs. LPS; 1-way ANOVA with Bonferroni's corrections (n=4). B) Adult male mice were treated with β2-agonist SAL. Survival was represented in a Kaplan-Meier analysis (n=20/group; P<0.01, survival log-rank test vs. control). Arrow represents the single dose of vehicle (control) or SAL (6 or 15 mg/kg i.p.) given 24 h after LPS challenge. C) Serum HMGB1 levels were analyzed by Western blot at 44 h after LPS challenge. Graph represents densitometric units (d.u.) from scanning of the film. *P < 0.01 vs. LPS; 1-way ANOVA with Bonferroni's corrections (n=5/group). D) Arrows represent 3 doses of vehicle (control) or SAL (15 mg/kg i.p.) started 24 h after CLP, and given every 12 h. Survival was analyzed and represented in a Kaplan-Meier analysis (n=15/group; P<0.01, survival log-rank test vs. control). E) VNS leads to the activation of the splenic nerve and subsequent release of norepinephrine (NE) in the spleen. Both NE (splenic nerve) and β2AR-agonists (salbutamol) inhibit cytokine production in the spleen and systemic inflammation in experimental sepsis through a mechanism specifically mediated by the β2-adrenoceptors of CD3+CD4+CD25− lymphocytes. This cellular mechanism resembles the innervation of the periarterial lymphoid sheath (PALS), where the splenic nerve makes synaptic-like connections with T lymphocytes located in the vicinity of macrophages (37).