Abstract
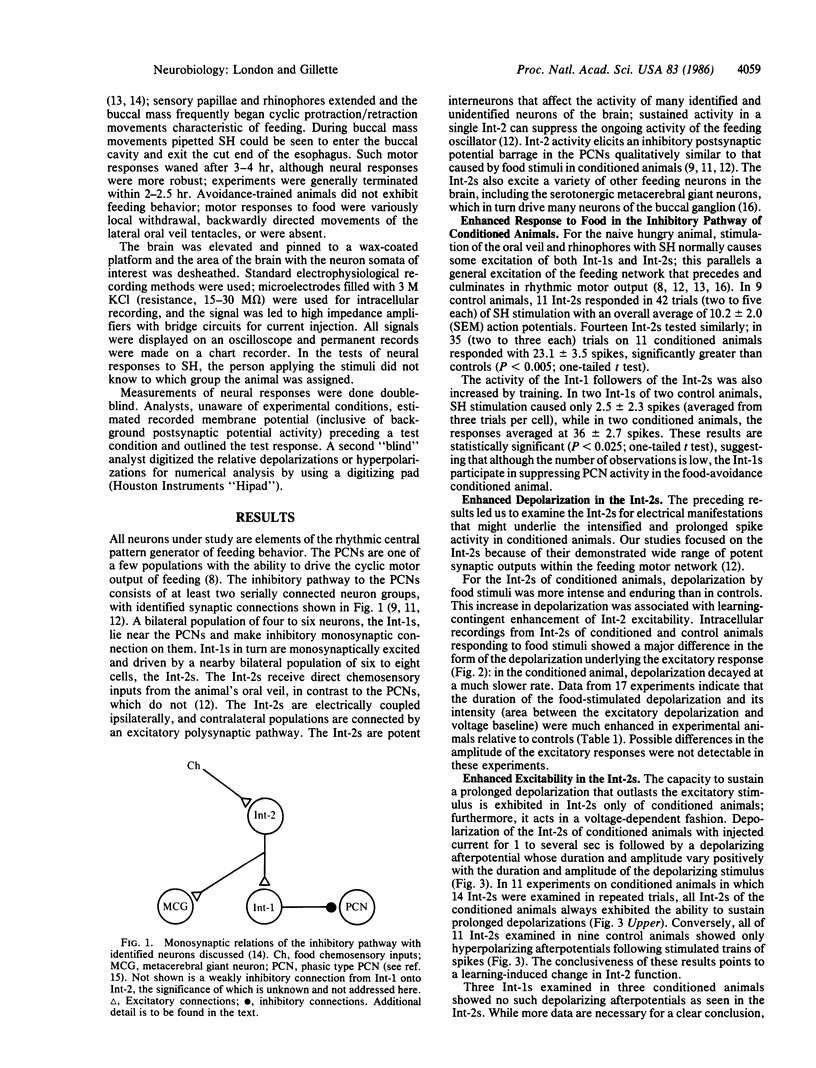
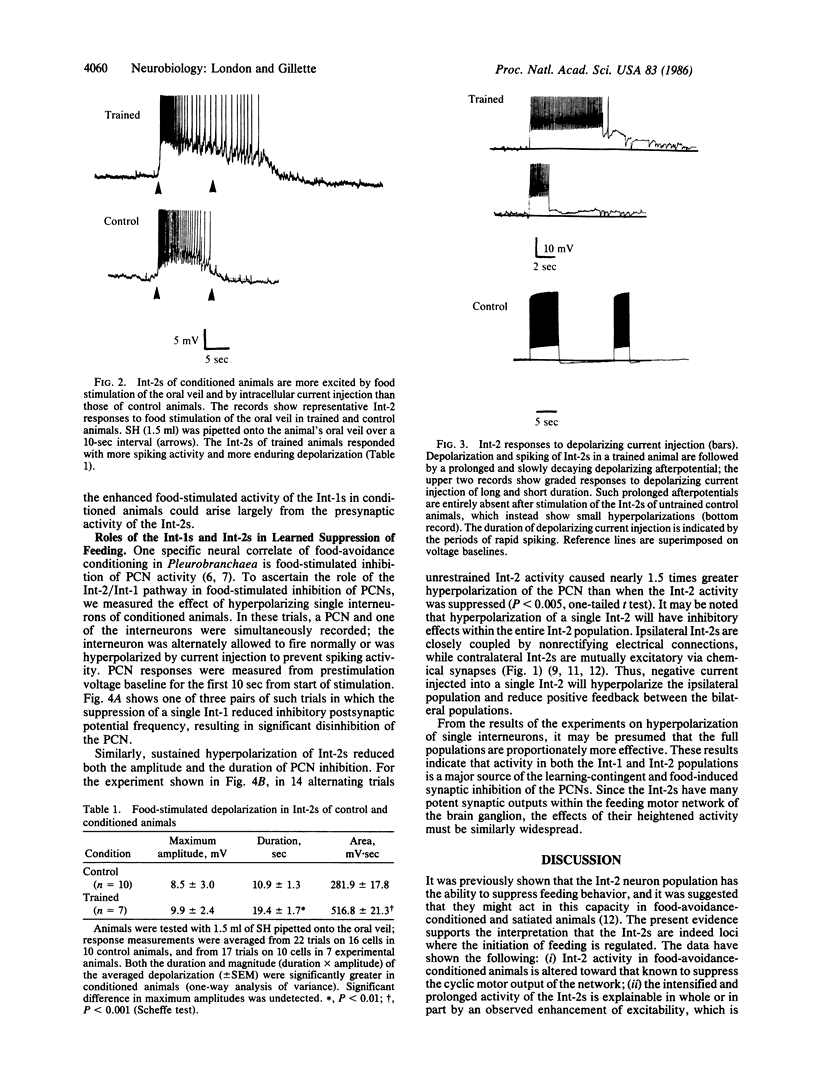
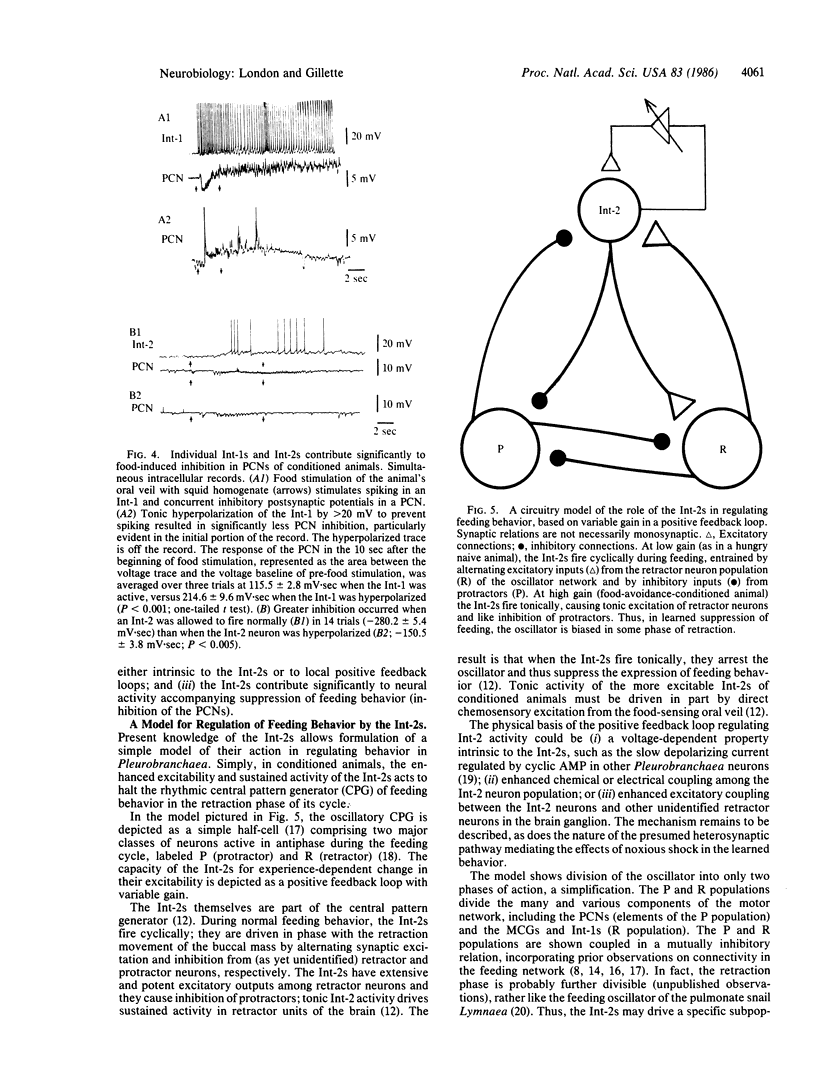
Food-avoidance conditioning in the mollusk Pleurobranchaea results in suppression of the feeding response to food stimuli. In conditioned animals, identified interneurons of the central pattern generator (CPG) for feeding behavior, the Int-2s, respond to a food stimulus with greater and more long-lasting excitation than controls. Enhanced Int-2 responsiveness to food stimuli is associated with markedly heightened Int-2 excitability. Sustained activity in the Int-2s arrests motor output of the oscillatory CPG in the protraction/retraction movement cycle of feeding through tonic excitation of a population of retractor interneurons and inhibition of protractors. The CPG locus of the learning mechanism is permissive of sensory excitation of alternative behavior and leaves the possibility open for release of the suppressed behavior in a fully aroused state.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crow T. J., Alkon D. L. Associative behavioral modification in hermissenda: cellular correlates. Science. 1980 Jul 18;209(4454):412–414. doi: 10.1126/science.209.4454.412. [DOI] [PubMed] [Google Scholar]
- Davis W. J., Gillette R., Kovac M. P., Croll R. P., Matera E. M. Organization of synaptic inputs to paracerebral feeding command interneurons of Pleurobranchaea californica. III. Modifications induced by experience. J Neurophysiol. 1983 Jun;49(6):1557–1572. doi: 10.1152/jn.1983.49.6.1557. [DOI] [PubMed] [Google Scholar]
- Davis W. J., Gillette R. Neural correlate of behavioral plasticity in command neurons of Pleurobranchaea. Science. 1978 Feb 17;199(4330):801–804. doi: 10.1126/science.622572. [DOI] [PubMed] [Google Scholar]
- Delcomyn F. Neural basis of rhythmic behavior in animals. Science. 1980 Oct 31;210(4469):492–498. doi: 10.1126/science.7423199. [DOI] [PubMed] [Google Scholar]
- Gillette M. U., Gillette R. Bursting neurons command consummatory feeding behavior and coordinated visceral receptivity in the predatory mollusk Pleurobranchaea. J Neurosci. 1983 Sep;3(9):1791–1806. doi: 10.1523/JNEUROSCI.03-09-01791.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette R., Kovac M. P., Davis W. J. Command neurons in Pleurobranchaea receive synaptic feedback from the motor network they excite. Science. 1978 Feb 17;199(4330):798–801. doi: 10.1126/science.622571. [DOI] [PubMed] [Google Scholar]
- Green D. J., Gillette R. Patch- and voltage-clamp analysis of cyclic AMP-stimulated inward current underlying neurone bursting. Nature. 1983 Dec 22;306(5945):784–785. doi: 10.1038/306784a0. [DOI] [PubMed] [Google Scholar]
- Hawkins R. D., Abrams T. W., Carew T. J., Kandel E. R. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science. 1983 Jan 28;219(4583):400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- Kovac M. P., Davis W. J., Matera E. M., Croll R. P. Organization of synaptic inputs to paracerebral feeding command interneurons of Pleurobranchaea californica. II. Inhibitory inputs. J Neurophysiol. 1983 Jun;49(6):1539–1556. doi: 10.1152/jn.1983.49.6.1539. [DOI] [PubMed] [Google Scholar]
- London J. A., Gillette R. Functional roles and circuitry in an inhibitory pathway to feeding command neurones in Pleurobranchaea. J Exp Biol. 1984 Nov;113:423–446. doi: 10.1242/jeb.113.1.423. [DOI] [PubMed] [Google Scholar]
- Mpitsos G. J., Collins S. D. Learning: rapid aversive conditioning in the gastropod mollusk Pleurobranchaea. Science. 1975 May 30;188(4191):954–957. doi: 10.1126/science.1138366. [DOI] [PubMed] [Google Scholar]
- Mpitsos G. J., Collins S. D., McClellan A. D. Learning: a model system for physiological studies. Science. 1978 Feb 3;199(4328):497–506. doi: 10.1126/science.622551. [DOI] [PubMed] [Google Scholar]
- Perkel D. H., Mulloney B. Motor pattern production in reciprocally inhibitory neurons exhibiting postinhibitory rebound. Science. 1974 Jul 12;185(4146):181–183. doi: 10.1126/science.185.4146.181. [DOI] [PubMed] [Google Scholar]
- Walters E. T., Byrne J. H. Associative conditioning of single sensory neurons suggests a cellular mechanism for learning. Science. 1983 Jan 28;219(4583):405–408. doi: 10.1126/science.6294834. [DOI] [PubMed] [Google Scholar]
- Weiss K. R., Cohen J., Kupfermann I. Potentiation of muscle contraction: a possible modulatory function of an identified serotonergic cell in Aplysia. Brain Res. 1975 Dec 5;99(2):381–386. doi: 10.1016/0006-8993(75)90041-4. [DOI] [PubMed] [Google Scholar]


