Abstract
1H-irradiation under mismatched Hartmann-Hahn conditions provides an alternative mechanism for carrying out 15N/13C transfers in triple-resonance heteronuclear correlation spectroscopy (HETCOR) on stationary samples of single crystals and aligned samples of biopolymers, which improve the efficiency especially when the direct 15N-13C dipolar couplings are small. In many cases, the sensitivity is improved by taking advantage of the 13Cα labeled sites in peptides and proteins with 13C detection. The similarities between experimental and simulated spectra demonstrate the validity of the recoupling mechanism and identify the potential for applying these experiments to virus particles or membrane proteins in phospholipid bilayers; however, further development is needed in order to derive quantitative distance and angular constraints from these measurements.
Keywords: 13C NMR, solid-state NMR, oriented samples, cross-polarization, triple-resonance
Introduction
Solid-state NMR spectroscopy is playing an increasingly important role in the determination of the structures of proteins in biological supramolecular assemblies, such as membrane proteins in phospholipid bilayers[1–4], coat proteins of virus particles[5], aggregates of amyloid proteins[6], etc. Oriented Sample (OS) solid-state NMR is particularly well suited to molecular assemblies that can be mechanically or magnetically[7] aligned in the field. This allows angular constraints to be derived from the measured frequencies relative to a single external axis, which fully defines the alignment tensor, unlike the situation for residual dipolar couplings (RDCs) in solution NMR where determination of the alignment tensor can be a major source of error [8]. Significantly, since each measurement is independent of the others on the molecule, any experimental errors in the measurements of frequencies or uncertainties in the magnitudes or molecular orientations of the spin-interaction tensors do not accumulate. This results in high-resolution and accurate structure determinations.
OS solid-state NMR has been successfully applied to DNA, membrane proteins, and viral coat proteins, primarily through the use of uniform and selective 15N labeling. 15N labeling of biopolymers has many advantages[9]. Uniform 15N labeling is easy and inexpensive to implement by expressing the protein or nucleic acid of interest in bacteria grown on chemically defined media where there is only a single source of nitrogen, typically a salt of ammonia. Since no nitrogens are directly bonded in biopolymers, and in the critical polypeptide backbone of proteins each amide nitrogen is separated from another by two carbon atoms and three bonds, homonuclear 15N/15N decoupling is not necessary at any stage of the experiments because of the combination of the low gyromagnetic ratio and spatial separation of nitrogen atoms. Many double-resonance 1H/15N experiments have been developed to measure frequencies from the three spin-interactions available at a single 15N labeled site: 1H chemical shift, 15N chemical shift, and 1H/15N heteronuclear dipolar couplings[10–13]. A number of assignment schemes have been developed based on both through-space interactions and the regularity of structural and spectral features that accompany the mapping of the structure onto the spectra by the anisotropic spin-interactions in the secondary structures of the α-helix and β-sheet[14; 15]. Recently, through-space methods of identifying proximate nuclei have been improved by invoking assistance from a third spin[16; 17].
In order to further advance OS solid-state NMR methods, triple-resonance experiments on 13C and 15N double labeled samples are an essential next step. This would enable spectroscopic interrogation of essentially all sites in a biopolymer. Since all backbone sites of a protein would be labeled it offers the possibility of systematic assignment schemes, and with 13C detection higher sensitivity. However, in fully labeled biomolecules there is a significant problem. The 13C form a dense network of homonuclear dipole-dipole coupled nuclei that interfere with most multidimensional solid-state NMR experiments as well as 13C detection. We have taken two approaches to ameliorating these difficulties. One is to use ‘tailored’ 13C labeling through judicious choice of 13C-containing precursors in the growth media[18]. This enables either uniform dilution of the 13C nuclei or high levels of labeling at selected sites where nearby carbons are unlabeled. The strong homonuclear dipole-dipole couplings are attenuated by the effect of the dilution on their spatial proximity. The second approach is to develop NMR experiments that incorporate homonuclear decoupling on both the 1H and 13C channels[19]. The implementation of triple-resonance experiments provides 13C chemical shift, and 1H-13C angular constraints that complement those from 15N especially since some of them are out of the peptide plane, and as a result add unique information to the structure calculations. Moreover, 13C detection offers increased sensitivity compared to the corresponding 15N detection.
Cross-polarization (CP) between abundant spins and dilute spins has been demonstrated successfully both in magic angle spinning (MAS) and stationary solid-state NMR experiments. The magnetization can be transferred from 1H to either 15N or 13C easily because of the strong dipolar couplings, up to 11 kHz or 22 kHz in peptides, respectively. In the basic cross-polarization experiment introduced by Waugh and coworkers[20], the observed signals are enhanced by up to ten- or four-fold for 15N and 13C, respectively[21]. Here we are interested in using these large initial increases in magnetization to enable subsequent transfers to provide additional frequency dimensions for resolution, and to initiate the development of systematic assignment methods for OS solid-state NMR.
The first goal of the pulse sequence is to transfer magnetization from the initially polarized dilute spin to the second type of dilute spin. In general, this is referred to as double-cross-polarization (DCP)[22], although the details of the spectroscopy can vary significantly among the pulse sequences used to carry out this procedure. In the simplest example, the magnetization can be transferred from 1H to 15N, and then from 15N to 13C; alternatively, the magnetization can be transferred from 1H to 13C, and then from 13C to 15N. In general, the efficiency of the magnetization transfer between dilute spins (15N and 13C) is low due to the relatively small dipolar couplings between these two nuclei, which are generally less than 1 kHz because of their relatively low gyromagnetic ratios.
Here we demonstrate that it is possible to obtain two-dimensional 13C/15N HETCOR spectra for all directly bonded pairs of 15N and 13Cα in the backbone of a peptide or protein. Ideally, we would like all of the correlation resonances to have equal intensity, however, for now we have to settle for reliable detection of the correlation resonances for all pairs of 15N and 13Cα in the peptides or proteins. Since the input for the structure calculations is in the form of the orientationally-dependent frequencies, not intensities or line shapes, this is much less of a handicap than in other classes of experiments, such as spin-exchange where the intensities of the off-diagonal peaks are important.
Longer mixing times generally improve the extent of magnetization transfer, especially when the dipolar couplings are small. However, the longer mixing times can also result in non-selective magnetization transfer or spin diffusion, depending on the various laboratory and rotating relaxation times. For solid-state NMR of stationary samples, selective magnetization transfers between 1H and 15N or 1H and 13C are feasible when the homonuclear 1H/1H dipole-dipole couplings are strongly attenuated, allowing individual heteronuclear dipolar couplings to dominant during specified time intervals of multi-dimensional experiments. Polarization inversion spin exchange at the magic angle (PISEMA),[10] SAMMY,[11] and related pulse sequences are able to decouple the homonuclear 1H dipolar networks and selectively transfer the magnetization between 1H and 15N[23; 24]. Alternatively, dipolar-based INEPT[25], also is effective for transferring magnetization when the 1H network of homonuclear couplings is suppressed. However, during many trials, we were unable to obtain efficient magnetization transfer between 15N and 13C using this family of pulse sequences. In contrast, spin-lock on both 15N and 13C with matched continuous wave irradiation does transfer magnetization between coupled 15N and 13C sites.
Third-spin assisted polarization transfer (TSAR) has been proposed as a method to recouple dilute spins under MAS condition[26]. However, instead of spinning the sample, the offsets can be created under the mismatched Hartmann-Hahn conditions between the abundant and dilute spins[16; 17] that effect recoupling in stationary solid-state NMR experiments, as shown in Equation1:
| (1a) |
| (1b) |
| (1c) |
Where S(1) and S(2) represent the dilute spins (15N and 13C), and I is 1H. ΔωI is the mismatched Hartmann-Hahn condition that makes the Hamiltonians significant when the mismatch is small. The first term, the recoupling term, recouples two dilute spins by the 1H, and therefore, the 1H dipolar network is used to create couplings among dilute spins. It is this alternative pathway that provides an opportunity to transfer magnetization when the dipolar couplings between dilute spins are weak. The second term, the equilibrating bath term, enable the equilibration of the spin temperature among the spins. For spin diffusion driven by this mechanism[16], 15N is polarized to achieve higher spin temperatures, and then, a z-filter is applied to eliminate any residual 1H magnetization that could result in non-selective transfer. Hence, the spin temperature is always higher for the 15N spins, and that drives the magnetization to redistribute to the proton bath and results in decreasing the transfer efficiencies. The third term can be neglected when the lattice sum of each dilute spin is equal; otherwise, the magnetization will be brought to the orthogonal frame according to the commutator. This term could decrease the transfer efficiency in the heteronuclear correlation experiments, because it is difficult to make the lattice sums of 13C and 15N equal to each other. Spin diffusion experiments among 15N in OS solid-state NMR with 1H-irradiation under mismatched Hartmann-Hahn conditions show that even if several different spin dynamics are involved, the recouping term still can assist the magnetization transfers. The Hamiltonians also suggest the feasibility of heteronuclear correlations in triple-resonance experiments on stationary samples. Here we demonstrate 13C-detected HETCOR experiments where the 15N/13C transfer is assisted by 1H-irradiation under mismatched Hartmann-Hahn conditions. In addition, we show that the enhancement of signal intensities and the selectivity depend upon experimental conditions.
Results and Discussion
Improvement in 13C/15N heteronuclear correlation spectra results from 1H-irradiation under mismatched Hartmann-Hahn conditions. This is demonstrated with two samples under stationary conditions. One sample is a single crystal of 15N, 13Cα N-acetyl-leucine (NAL), which has four unique molecules in its unit cell. The other sample is selectively 15N, 13Cα alanine-labeled Pf1 bacteriophage coat protein in magnetically aligned virus particles. There are 8 alanine residues in the protein sequence.
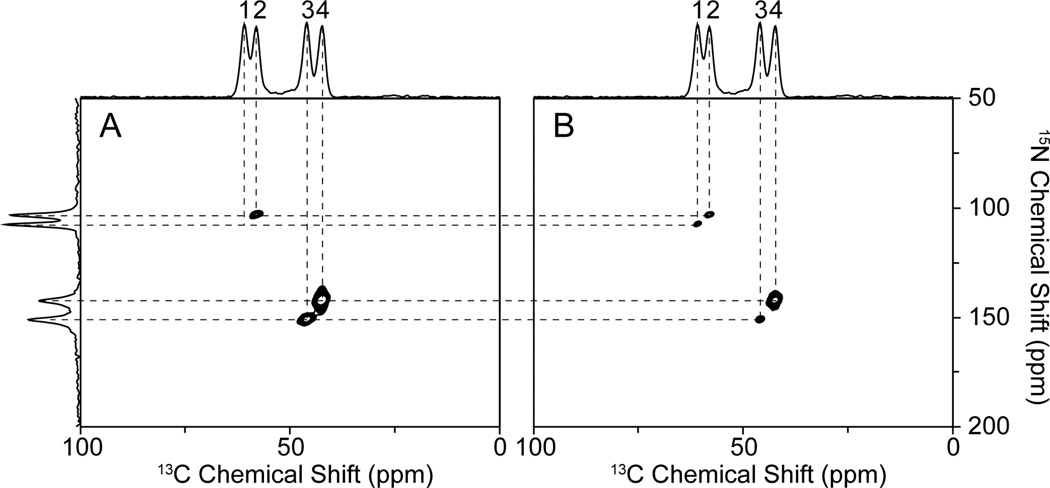
One-dimensional 13C and 15N NMR spectra obtained by conventional spin-lock cross-polarization are shown in Figure 2A and B; the four resolved resonances in each spectrum correspond to the four molecular orientations in the unit cell of the single crystal. The 15N-13C heteronuclear dipolar coupling doublets generated in the t1 dimension by the separated-local-field (SLF) experiment using the pulse sequence diagramed in Figure 1A are shown in Figure 2C and D. Both the chemical shift and heteronuclear dipolar coupling frequencies depend on the angles between 15N-13Cα bonds and the magnetic field. As a result, the 15N and 13Cα sites in a single peptide plane have the same 15N-13C dipolar coupling. By matching the 15N-13C dipolar couplings in the 15N- and 13C-detected 15N-13C dipolar coupling SLF spectra, all four resonances in the 15N and 13C one-dimensional spectra can be correlated. The 15N and 13Cα pairs are identified in Figure 2. The 15N-13Cα dipolar couplings are 200 Hz, 374 Hz, and 451 Hz for pair 2, pair 3 and pair 4, respectively. These doublets show the 15N-13C dipolar couplings are small, as predicted for the peptide system. The 15N-13Cα dipolar coupling of pair 1 is below the resolution limit of this particular experiment, and therefore we classify the heteronuclear dipolar coupling of this pair to be ~ 0 kHz.
Figure 2.
13C and 15N chemical shifts correlated by their 15N-13C heteronuclear dipolar couplings in SLF spectra of a 15N, 13Cα NAL single crystal. A. 15N decoupled 13C NMR spectrum. B. 13C decoupled 15N NMR spectrum. C. 13C-detected 15N-13C heteronuclear dipolar coupling SLF spectra. D. 15N-detected 15N-13C heteronuclear dipolar coupling SLF spectra. The observed 15N-13Cα heteronuclear dipolar couplings are: ~0 Hz (pair 1); 200 Hz (pair 2); 374 Hz (pair 3); and 451 Hz (pair 4).
Figure 1.
Timing diagrams for the pulse sequences. A. 15N-13C dipolar coupling / 13C chemical shift or 15N-13C dipolar coupling / 15N chemical shift separated-local-field (SLF) experiment. B. 15N/13C HETCOR experiment. C. 15N/13C HETCOR experiment with 1H-irradiation. SLF experiments provide the local fields (dipolar couplings) affecting on the observed spins in the t1 dimension, and the pulse scheme in the t1 dimension can be modified for different demands (1H-15N, 1H-13C, or 15N-13C dipolar couplings). Therefore, the chemical shifts acquired in the t2 dimension can be resolved by these two-dimensional experiments[31; 32]. CW refers to continuous wave irradiation and SPINAL-16[33] refers to the modulation used for heteronuclear decoupling. Pre-saturation is accomplished with thirty 90° pulses separated by 200 µs delay. The z-filter is 5 ms. Mix time for 1H/15N cross-polarization is 1ms, and for 15N/13C cross-polarization is 1ms or 3ms.
The conventional 15N/13C HETCOR experiments were performed using the pulse sequence in Fig. 1B. 15N and 13C are pre-saturated to ensure that the magnetization is transferred from other spins without interference from residual magnetization. The magnetization is first transferred from 1H to 15N, and the 15N nuclei then evolve according to their chemical shift interactions by removing heteronuclear dipolar couplings. Subsequently, a 5ms z-filter is applied to remove any residual 1H magnetization. The first 90° pulse brings the 15N magnetization to the z-axis, and is alternated between −Y and −X phase to achieve quadrature detection in the t1 dimension; the second 90° pulse brings the magnetization back, and is alternated between Y and −Y phase to suppress the effects of probe ringing. The magnetization is subsequently transferred with the continuous wave irradiation applied on the 15N and 13C channels simultaneously, and 13C chemical shifts are detected in the t2 dimension. Three resonances shown in Fig. 3A were obtained in the regular 15N/13C HETCOR experiment with 1ms 15N/13C mixing time. The missing resonance corresponds to resonance 1, which has ~ 0 kHz 15N-13C dipolar coupling. Notably, no magnetization transfer is observed for pair 1 even with the mixing time extended to 3 ms.
Figure 3.
A. 13C-detected 15N/13C HETCOR spectrum of a 15N, 13Cα NAL single crystal. B. 13C-detected 15N/13C HETCOR with 1H-irradiation at 40 kHz. The one-dimensional spectra aligned on the top and side are the same 13C and 15N CP spectra shown in Figure 2A and B, respectively. Note in Figure 3B that the signal from pair 1 is detectable only in the presence of 1H-irradiation. The experiments were performed with 1ms (shown) and 3ms 15N/13C mixing time with similar results.
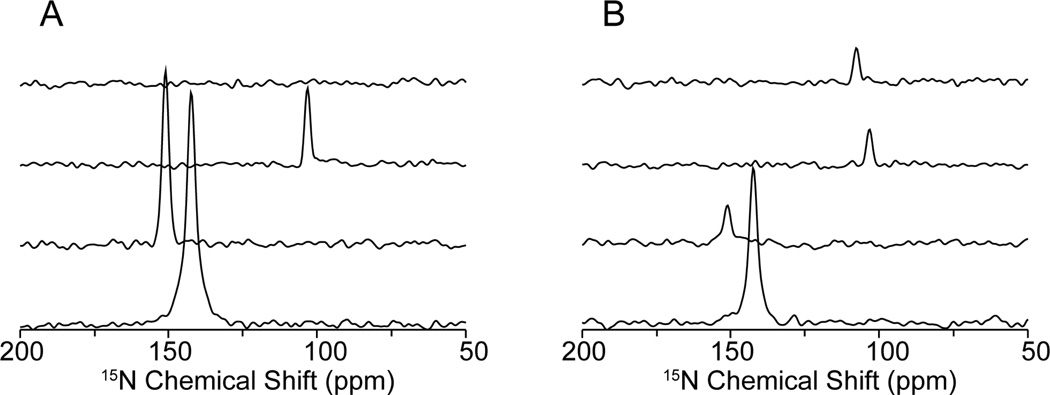
Four resonances are present in Fig. 3B with 1H-irradiation at 40 kHz during the 15N/13C mixing period (Fig. 1B). Slices through all the resonances in the t1 dimension are shown in Fig. 4. The experimental results on the single crystal are fully consistent with the described model: 1H-irradiation provides another pathway to transfer the magnetization from 15N to 13Cα via the proton bath. The weaker intensities of the pair 2 to pair 4 imply that the other two terms (Equation 1b and c) are involved as well and result in loss of signal intensity. The benefit of 1H-irradiation is shown on pair 1. Although, there are other processes competing with recoupling, the experimental results show that recoupling can drive the magnetization transfer without direct dipolar couplings. Non-selective transfer from the proton bath when 1H-irradiation is applied can be ruled out by inspecting the slices in Fig. 4, since the sharp peaks and flat baselines show only that a specific frequency is transferred.
Figure 4.
One-dimensional spectral slices along the 15N chemical shift dimension taken from the two-dimensional spectra shown in Figure 3. A. 15N/13C HETCOR. B. 15N/13C HETCOR with 1H-irradiation at 40 kHz. From top to bottom, the slices correspond to pairs 1 to 4, respectively. In Figure 4A, the measured signal-to-noise ratios are 36 (pair 2), 83 (pair 3) and 111 (pairs 4). In Figure 4B, the measured signal-to-noise ratios are 17 (pair 1), 18 (pair 2), 18 (pair 3) and 74 (pair 4). Reductions in some signal intensities result from competing pathways. The narrow single-line peaks and the flat baselines indicate that the transfer from 15N to 13C is selective.
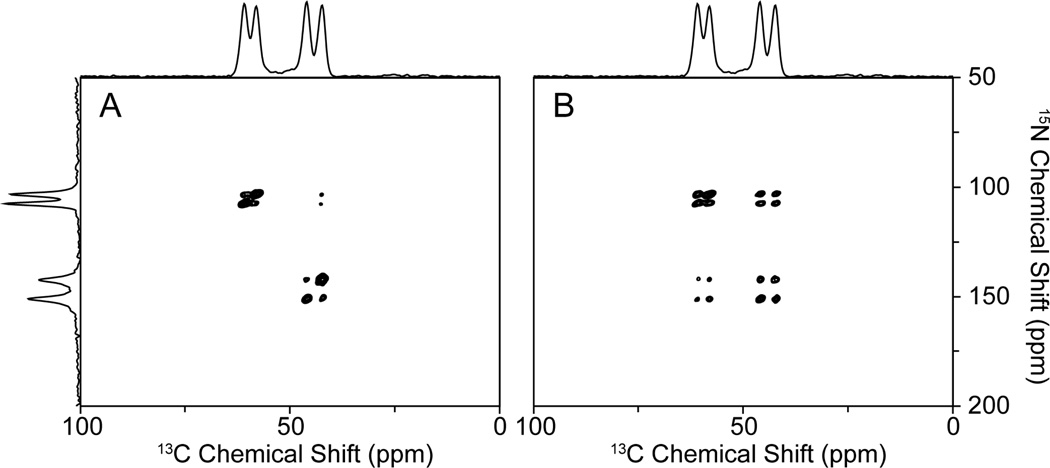
Recoupling has been shown to assist the 15N/13C transfers when the mismatches are lower than the Hartmann-Hahn condition by 10 kHz. According to the Hamiltonians, the same effect should occur when the mismatch is higher as well. The stronger 1H-irradiation brings the 1H magnetization into the rotating frame more efficiently, and should provide superior recoupling; once the proton bath recouples the dilute spins, all the correlations between 15N and 13C should be observed in the HETCOR spectra. However, we only observed the intra-molecular correlations in the 15N, 13Cα NAL single crystal sample in the presence of 1H-irradiation at 40 kHz. When the field strength of 1H-irradiation was increased to 60 kHz not only the intra- but also some inter-correlations were observed in the spectra (Fig. 5A). Comparing the spectrum in Fig. 3B, the only difference is the strength of 1H-irradiation, and it shows that the recoupling efficiency is improved by increasing the strength of the B1 field. All the correlations expected in the unit cell were observed when mixing time was extended to 3ms (Fig. 5B).
Figure 5.
A. and B. 15N/13C HETCOR spectra obtained with 1H-irradiation of 60 kHz with 1ms and 3ms 15N/13C mixing times, respectively, of a 15N, 13Cα NAL single crystal. One-dimensional spectra on the top and the side are the 13C and 15N CP spectra shown in Figure 2 A and B. Strong inter-molecular correlations in a single crystal are achieved only under the higher mismatched condition.
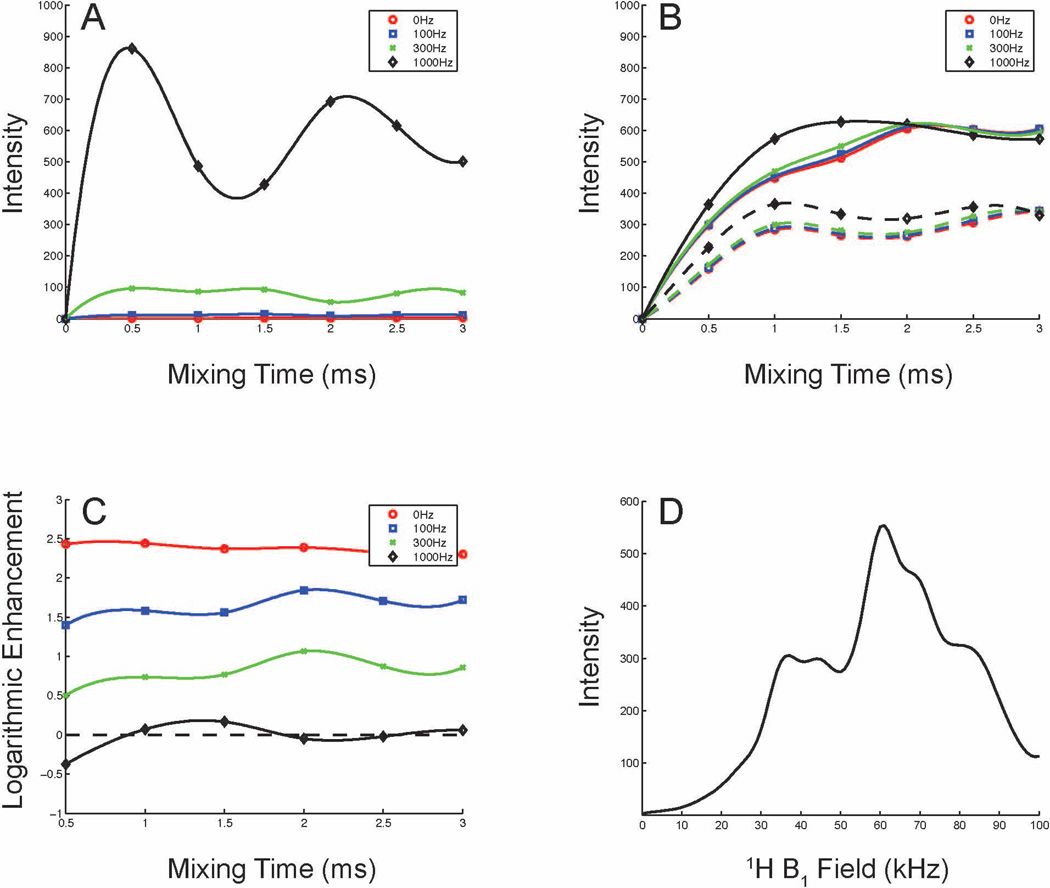
Simulations of the magnetization transfer in a 15N, 13Cα NAL peptide were carried out using SIMPSON 3.0.1[27]. To simplify the spin system, only 1H nuclei attached to the 15N, Cα, Cβ, and N-methyl groups were considered, and only the 15N/13C mixing period was simulated to evaluate the transfer efficiency. In the simulations, a 1kHz 15N-13C dipolar coupling was utilized. The regular 15N/13C transfer (Fig. 6A) is sensitive to 15N-13C dipolar couplings because they provide the only transfer pathway. The typical CP buildup curve[28] is observed in the simulations when the 15N-13C dipolar coupling is strong; the intensities increase proportionally to the magnitudes of the dipolar couplings, and the dipolar modulation also causes the oscillations in the magnetization buildup curve. The transfer efficiencies are poor when the 15N-13C dipolar couplings are small. However, in the presence of 1H-irradiation (Fig. 6B), no matter the magnitude of the dipolar couplings, they tend to have similar transfer efficiencies. Both higher and lower mismatched conditions (59 kHz and 43 kHz according to the mismatched profile in Fig. 6D) were simulated. The higher field provides better transfer efficiencies, which is consistent with the experimental results shown in Fig. 3B and Fig. 5. This demonstrates that the transfer mechanism is dominated by recoupling with the proton bath. The data in Fig. 6C show that transfers for weak 15N-13C dipolar couplings benefit from 1H-irradiation, but that it can decrease the efficiencies for strong 15N-13C dipolar couplings. The mismatched profile (Fig. 6D) was simulated under the small dipolar coupling condition. Two local maxima show up asymmetrically when mismatched conditions are about ±10 kHz, which is similar to the simulations of 1H-irradiation assisted 15N spin diffusion, where the optimal mismatched conditions occur at ± 8%[16]. Both the experimental results and simulations suggest that the optimal conditions for 1H-irradiation lie between ±5 kHz and ±15 kHz, mainly depending on the properties of the proton dipolar network. Better transfer efficiency can be observed at the higher mismatch condition because of the larger 1H magnetization in the rotating frame. In practice, the higher mismatched condition drives the inter-peptide correlations that distribute the magnetizations to the proton bath and more distant dilute spins. As a result, the transfer efficiencies would be decreased as shown in Fig. 5.
Figure 6.
Simulations of the transfer efficiencies between 15N and 13C with various 15N-13C heteronuclear dipolar couplings. A. Conventional 15N/13C CP transfer. B. 15N/13C CP transfer with 1H-irradiation at 59 kHz (solid line) and 43 kHz (dashed line). C. Logarithmic plot of enhancement by 1H-irradiation. D. Mismatched profile of 1H-irradiation. A. - C. the 15N-13Cα dipolar couplings were simulated for 0 (red), 100 (blue), 300 (green), 1000 (black) Hz with all other parameters kept the same. The buildup curves were sampled every 500 ms, and the lines connecting the data points show the trends. D. The simulation was done when 15N-13Cα dipolar coupling equal to zero, and the strength of 1H-irradiation was varied from 0 kHz to 100 kHz.
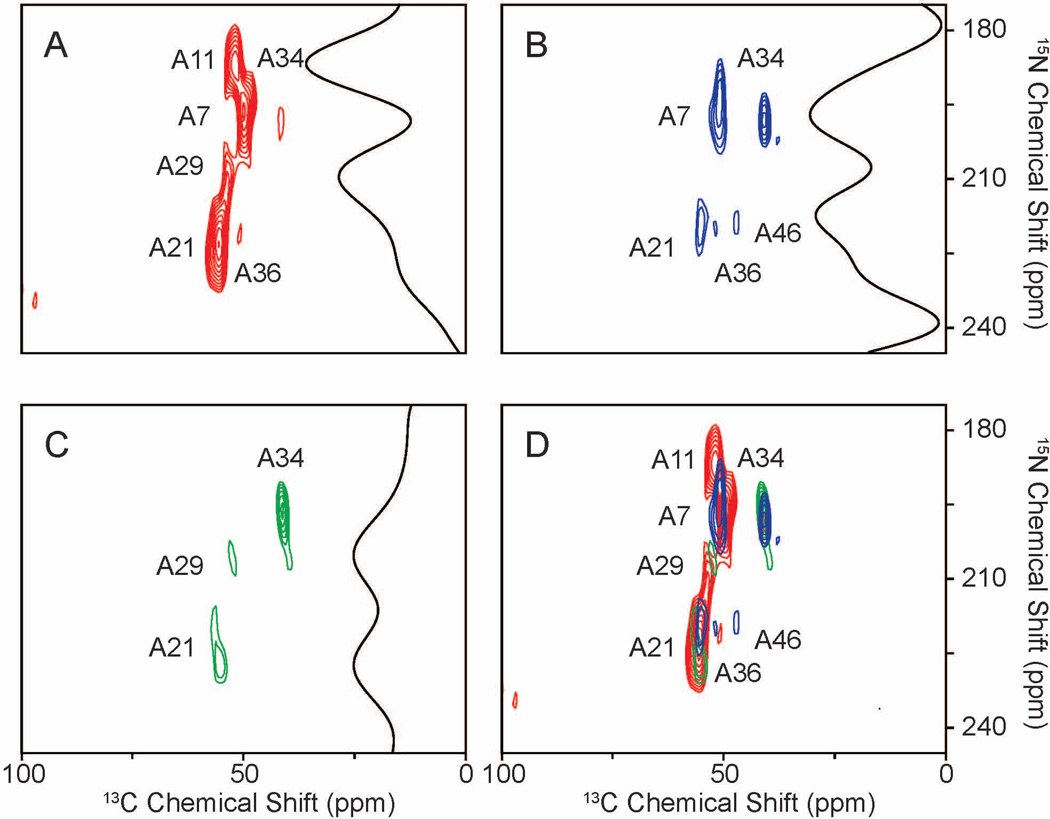
15N, 13Cα alanine-labeled Pf1 bacteriophage was used to demonstrate the feasibility for the applications to aligned protein samples. The experiments were performed at a relatively low temperature, to ensure that a single conformation of the protein was present. [29]. All the resonances were assigned by comparisons to previous results. Signals from seven out of the eight alanine residues were observed with the regular HETCOR experiment (Fig. 7A). A7, A11, and A21 are strong and well resolved. A29 is merged with A21, and A34 and A36 are relatively weak comparing to the other peaks. The signal from A46 is not observed. With 1H-irradiation at a lower mismatched condition of 33.8 kHz, two changes are apparent in the spectrum in Fig. 7B: the signal-to-noise ratio from A34 is increased by 50%, and the signal from A46 can be observed. For the higher mismatched condition at 67.7 kHz, A34 still gains 50% enhancement, while A21 is significantly reduced, which distinguishes between A21 and A29 in Fig. 7C. Other peaks were reduced in intensity in the presence of 1H-irradiation, which serves to reinforce the agreement between the single crystal spectra and simulations. The superimposed spectrum shown in Fig. 7D contains all resonances that are expected from 15N, 13Cα alanine-labeled Pf1 bacteriophage. Thus be performing multiple experiments with varying strengths of 1H-irradiation, it is possible to detect all 15N/13C correlation peaks from a protein, despite their widely varying magnitudes of heteronuclear dipolar couplings.
Figure 7.
Two-dimensional 15N/13C HETCOR spectra of selectively 15N, 13Cα alanine-labeled Pf1 bacteriophage that is magnetically aligned in the field. The protein has eight alanine residues. A. Two-dimensional 15N/13C HETCOR without 1H-irradiation. B. Two-dimensional 15N/13C HETCOR with 1H-irradiation at 33.8 kHz. C. Two-dimensional 15N/13C HETCOR with 1H-irradiation at 67.7 kHz. The slices shown in panels A., B., and C. were taken at 53.7 ppm in the 13C chemical shift dimension, corresponding to the resonance from A29. D. This spectrum is a superposition of the spectra in panels A.(red), B.(blue), and C.(green); it displays all of the 15N/13C correlations in the selectively 15N- alanine labeled Pf1 bacteriophage, and demonstrates the possibility of observing all 15N/13C correlations regardless of the strength of their heteronuclear dipolar couplings by obtaining spectra under several different transfer conditions.
Conclusions
13C-detected triple-resonance experiments can detect all 15N/13C correlation resonances with high sensitivity, as long as they are performed with 13C-detection under several different conditions of 1H radiofrequency irradiations. The frequencies of the 15N/13C correlation resonances provide valuable constraints for the calculation of protein structures in OS solid-state NMR. Moreover, 15N/13C transfers can be used as a filter to eliminate interference from natural abundance signals from the lipids [18]. 1H-irradiation under mismatched Hartmann-Hahn conditions assists magnetization transfer from 15N to 13C. The experimental results obtained on single crystal and aligned protein samples, and simulations demonstrate the validity of this recoupling mechanism. The transfer mechanism relies primarily on the 1H dipolar network, which is well suited for OS solid-state NMR experiments. The spin dynamics are complex once the spins are recoupled by 1H-irradiation, and therefore, some of the transfer efficiencies may be lost, especially when the 15N-13C dipolar couplings are large. However, this method is complementary to other procedures especially because it provides good transfer efficiencies for small 15N-13C dipolar couplings. Both mismatched conditions are used in order to enhance the intensities of signals with different dipolar couplings. Combined with conventional spin-lock CP transfer, these experiments have the potential to simultaneous measure all of the 13C and 15N chemical shift frequencies and correlate proximate residues in a protein, which provides the basis for systematic assignment schemes.
Methods
15N, 13Cα NAL single crystal spectra
The NMR experiments were performed on a Varian Inova spectrometer with 1H, 13C, and 15N frequencies of 500.125 MHz, 125.76 MHz, and 50.68 MHz, respectively. A home-built modified Alderman Grant coil (MAGC) triple-resonance probe[19], which was designed for lossy biological samples, was used for these experiments. All the spectra were obtained at room temperature. The 1H carrier frequency was set at 4.7 ppm; the 13C carrier frequency was set at 54.94 ppm; and the 15N carrier frequency was set at 79.26 ppm. 13C- and 15N-detected spectra were acquired with 512 and 1024 complex points in the direct dimensions, respectively. CP spectra were acquired with 64 scans and 40 µs dwell time. 15N-13C dipolar coupling SLF spectra were acquired with 4 scans, 100 µs dwell time and 96 points in the indirect dimension, and 40 µs dwell time in the direct dimension. HETCOR spectra were acquired with 4 scans, 100 µs dwell time and 192 complex points in the indirect dimension, and 40 µs dwell time and 512 complex points in the direct dimension. The mixing time for 1H/15N and 1H/13C CP was 1ms, and 15N/13C CP was 1ms or 3ms. For all the experiments the recycle delay was 6 s. Pre-saturation consisted of thirty 90° pulses separated by 200 µs, and the z-filter delay was 5 ms. The strength of the continuous wave decoupling on 15N and 13C channels was 40 kHz, and all other irradiations were performed at 50 kHz, except for the variable 1H-irradiation during the 15N/13C mixing period. The experimental data were zero filled to 512 and 1024 data points in the indirect and direct dimensions, respectively, and were multiplied by a sine bell window function prior to Fourier transformation.
15N, 13Cα alanine-labeled Pf1 bacteriophage spectra
The Pf1 filamentous bacteriophage sample was prepared as described previously[30]. The final bacteriophage sample was diluted to 40 mg/ml with sodium borate buffer (pH=8), and the final volume was 150 µl. The spectra were obtained at −2°C to ensure that the coat protein was in its ‘low temperature’ conformation. The 1H carrier frequency was set at 4.7 ppm; the 13C carrier frequency was set at 100 ppm, and the 15N carrier frequency was set at 213 ppm. HETCOR spectra were acquired with 400 scans, 100 µs dwell time, 32 complex points in the indirect dimension, and 25 µs dwell time and 256 complex points in the direct dimension. Mixing time of 1H/15N CP was 1ms and 15N/13C CP was 3ms. The recycle delay was 4 s. All other conditions were the same as described above for the single crystal.
Simulations
Only the 15N/13C mixing period was simulated to evaluate the transfer efficiency. The simulations were carried out with SIMPSON 3.0.1. The model was based on the single crystal structure[16]; however, the spin system was simplified to 15N, 13Cα, the 1H attached to the N, Cα, Cβ, and N-methyl group. Euler angles “β” in the input file were manually adjusted to 90° for 1H-15N, 1H-13Cα and 15N-13Cα dipolar couplings, and therefore, the dipolar couplings can be varied without angular considerations. For all the simulations, 1H-15N and 1H-13Cα dipolar couplings were 12.18 and 22.80 kHz according to the bond lengths. 15N-13Cα dipolar couplings were adjusted manually according to each case. 15N/13C mixing period was 3 ms for the mismatched profile simulation.
Highlights.
Heteronuclear dipolar couplings differ by two in perpendicular and parallel bilayers
Dipolar couplings correlate resonances in perpendicular and parallel bilayers
Assignments result from the dipolar correlation and an assigned isotropic spectrum
Acknowledgements
We thank C. H. Wu and B. B. Das for helpful discussions. This research was supported by grants from the National Institutes of Health, and it utilized the Biomedical Technology Resource for NMR Molecular Imaging of Proteins at the University of California San Diego, which is supported by grant P41EB002031.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marassi FM, Ma C, Gratkowski H, Straus SK, Strebel K, Oblatt-Montal M, Montal M, Opella SJ. Correlation of the structural and functional domains in the membrane protein Vpu from HIV-1. Proc. Natl. Acad. Sci. U. S. A. 1999;96:14336–14341. doi: 10.1073/pnas.96.25.14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park SH, Prytulla S, De Angelis AA, Brown JM, Kiefer H, Opella SJ. High-resolution NMR spectroscopy of a GPCR in aligned bicelles. J. Am. Chem. Soc. 2006;128:7402–7403. doi: 10.1021/ja0606632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Angelis AA, Howell SC, Nevzorov AA, Opella SJ. Structure determination of a membrane protein with two trans-membrane helices in aligned phospholipid bicelles by solid-state NMR spectroscopy. J. Am. Chem. Soc. 2006;128:12256–12267. doi: 10.1021/ja063640w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook GA, Opella SJ. NMR studies of p7 protein from hepatitis C virus. Eur. Biophys. J. 2009;39:1097–1104. doi: 10.1007/s00249-009-0533-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Opella SJ, Zeri AC, Park SH. Structure, dynamics, and assembly of filamentous bacteriophages by nuclear magnetic resonance spectroscopy. Annu. Rev. Phys. Chem. 2008;59:635–657. doi: 10.1146/annurev.physchem.58.032806.104640. [DOI] [PubMed] [Google Scholar]
- 6.Baryro MJ, Maly T, Birkett NR, Bacphee CE, CM D, Griffin RG. High-Resolution MAS NMR Analysis of PI3-SH3 Amyloid Fibrils: Backbone Conformation and Implications for Protofilament Assembly and Structure. Biochemistry. 2010;49:7474–7484. doi: 10.1021/bi100864t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Angelis AA, Nevzorov AA, Park SH, Howell SC, Mrse AA, Opella SJ. High-Resolution NMR Spectroscopy of Membrane Proteins in Aligned Bicelles. J. Am. Chem. Soc. 2004;126:15340–15341. doi: 10.1021/ja045631y. [DOI] [PubMed] [Google Scholar]
- 8.Tjandra N, Bax A. Direct Measurement of Distances and Angles in Biomolecules by NMR in a Dilute Liquid Crystalline Medium. Science. 1997;278:1111–1114. doi: 10.1126/science.278.5340.1111. [DOI] [PubMed] [Google Scholar]
- 9.Cross TA, DiVerdi JA, Opella SJ. Strategy for nitrogen NMR of biopolymers. J. Am. Chem. Soc. 1982;104:1759–1761. [Google Scholar]
- 10.Wu C, Ramamoorthy A, Opella S. High-resolution heteronuclear dipolar solid-state NMR spectroscopy. J. Magn. Reson. 1994;A109:270–272. [Google Scholar]
- 11.Nevzorov AA, Opella SJ. A "magic sandwich" pulse sequence with reduced offset dependence for high-resolution separated local field spectroscopy. J. Magn. Reson. 2003;164:182–186. doi: 10.1016/s1090-7807(03)00240-4. [DOI] [PubMed] [Google Scholar]
- 12.Wu CH, Opella SJ. Proton-detected separated local field spectroscopy. J. Magn. Reson. 2008;190:165–170. doi: 10.1016/j.jmr.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu CH, Opella SJ. Shiftless nuclear magnetic resonance spectroscopy. J. Chem. Phys. 2008;128:052312. doi: 10.1063/1.2816786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mesleh MF, Veglia G, DeSilva TM, Marassi FM, Opella SJ. Dipolar waves as NMR maps of protein structure. J. Am. Chem. Soc. 2002;124:4206–4207. doi: 10.1021/ja0178665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marassi FM, Opella SJ. A solid-state NMR index of helical membrane protein structure and topology. J. Magn. Reson. 2000;144:150–155. doi: 10.1006/jmre.2000.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nevzorov AA. Mismatched Hartmann-Hahn Conditions Cause Proton-Mediated Intermolecular Magnetization Transfer between Dilute Low-Spin Nuclei in NMR of Static Solids. Journal of the American Chemical Society. 2008;130:11282–11283. doi: 10.1021/ja804326b. [DOI] [PubMed] [Google Scholar]
- 17.Traaseth NJ, Gopinath T, Veglia G. On the Performance of Spin Diffusion NMR Techniques in Oriented Solids: Prospects for Resonance Assignments and Distance Measurements from Separated Local Field Experiments. J. Phys. Chem. B. 2010;114:13872–13880. doi: 10.1021/jp105718r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinha N, Filipp FV, Jairam L, Park SH, Bradley J, Opella SJ. Tailoring 13C labeling for triple-resonance solid-state NMR experiments on aligned samples of proteins. Magn. Reson. Chem. 2007;45 Suppl 1:S107–S115. doi: 10.1002/mrc.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin EC, Wu CH, Yang Y, Grant CV, Opella SJ. 1H-13C separated local field spectroscopy of uniformly 13C labeled peptides and proteins. J. Magn. Reson. 2010;206:105–111. doi: 10.1016/j.jmr.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pines A, Gibby MG, Waugh JS. Proton-enhanced nuclear induction spectroscopy. A method for high-resolution NMR of dilute spins in solids. J. Chem. Phys. 1972;56:1776–1777. [Google Scholar]
- 21.Pines A, Gibby M, Waugh J. Proton enhanced NMR of dilute spins in solids. J. Chem. Phys. 1973;59:569–590. [Google Scholar]
- 22.Schaefer J, McKay RA, Stejskal EO. Double-cross-polarization NMR of solids. J. Magn. Reson. 1979;34:443–447. [Google Scholar]
- 23.Ramamoorthy A, Wu CH, Opella SJ. Three-dimensional solid-state NMR experiment that correlates the chemical shift and dipolar coupling frequencies of two heteronuclei. J. Magn. Reson. 1995;B107:88–90. doi: 10.1006/jmrb.1995.1063. [DOI] [PubMed] [Google Scholar]
- 24.Nevzorov AA, Park SH, Opella SJ. Three-dimensional experiment for solid-state NMR of aligned protein samples in high field magnets. J. Biomol. NMR. 2007;37:113–116. doi: 10.1007/s10858-006-9121-y. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Soong R, Im S-C, Waskell L, Ramamoorthy A. INEPT-Based Separated-Local-Field NMR Spectroscopy: A Unique Approach To Elucidate Side-Chain Dynamics of Membrane-Associated Proteins. Journal of the American Chemical Society. 2010;132:9944–9947. doi: 10.1021/ja103983f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewandowski JR, De Paëpe G, Griffin RG. Proton Assisted Insensitive Nuclei Cross Polarization. J. Am. Chem. Soc. 2007;129:728–729. doi: 10.1021/ja0650394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bak M, Rasmussen J, Nielsen N. SIMPSON: a general simulation program for solid-state NMR spectroscopy. Journal of Magnetic Resonance. 2000;147:296–330. doi: 10.1006/jmre.2000.2179. [DOI] [PubMed] [Google Scholar]
- 28.Müller L, Kumar A, Baumann T, Ernst RR. Transient Oscillations in NMR Cross-Polarization Experiments in Solids. Phys. Rev. Lett. 1974;32:1402. [Google Scholar]
- 29.Thiriot DS, Nevzorov AA, Opella SJ. Structural basis of the temperature transition of Pf1 bacteriophage. Protein Sci. 2005;14:1064–1070. doi: 10.1110/ps.041220305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thiriot DS, Nevzorov AA, Zagyanskiy L, Wu CH, Opella SJ. Structure of the coat protein in Pf1 bacteriophage determined by solid-state NMR spectroscopy. J. Mol. Biol. 2004;341:869–879. doi: 10.1016/j.jmb.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 31.Waugh JS. Uncoupling of local field spectra in nuclear magnetic resonance: Determination of atomic position in solids. Proc. Natl. Acad. Sci. U. S. A. 1976;73:1394–1397. doi: 10.1073/pnas.73.5.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinha N, Grant CV, Park SH, Brown JM, Opella SJ. Triple resonance experiments for aligned sample solid-state NMR of (13)C and (15)N labeled proteins. J. Magn. Reson. 2007;186:51–64. doi: 10.1016/j.jmr.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinha N, Grant CV, Wu CH, De Angelis AA, Howell SC, Opella SJ. SPINAL modulated decoupling in high field double- and triple-resonance solid-state NMR experiments on stationary samples. J. Magn. Reson. 2005;177:197–202. doi: 10.1016/j.jmr.2005.07.008. [DOI] [PubMed] [Google Scholar]