Abstract
Background and objectives
Detection of peripheral fundus autofluorescence (FAF) using conventional scanning laser ophthalmoscopes (SLOs) is difficult and requires pupil dilation. Here we evaluated the diagnostic properties of wide-field FAF detected by a two-laser wavelength wide-field SLO in uveitis patients.
Study design/materials and methods
Observational case series of four patients suffering from different types of posterior uveitis/chorioretinitis. Wide-field FAF images were compared to visual fields. Panretinal FAF was detected by a newly developed SLO, which allows FAF imaging of up to 200° of the retina in one scan without the need for pupil dilation. Visual fields were obtained by Goldmann manual perimetry.
Results
Findings from wide-field FAF imaging showed correspondence to visual field defects in all cases.
Conclusion
Wide-field FAF allowed the detection of visual field defect-related alterations of the retinal pigment epithelium in all four uveitis cases.
Keywords: fundus autofluorescence (FAF), Optomap, wide-field scanning laser ophthalmoscopy, imaging, uveitis, visual field
Introduction
Detection of peripheral fundus autofluorescence (FAF) using conventional scanning laser ophthalmoscopes (SLOs) is often difficult, as most of these imaging devices only allow 30° to 60° of the retina to be captured in one scan. In addition, pupil dilation is usually required for adequate image quality.1
Recently, the nonmydriatic two-laser wavelength wide-field SLO Optomap (Optomap P200Tx; Optos PLC, Dunfermline, Fife, Scotland) has been developed. This novel device allows detection of FAF for up to 200° of the retina in one scan. Therefore, the Optomap P200Tx provides nonmydriatic imaging not only for the posterior pole of the retina, but even extending over the equator.2
The Optomap P200Tx SLO includes two scanning laser wavelengths: green (532 nm) and red (633 nm). For detection of FAF, the green laser is sent into the eye and used as an exciter. Certain fluorophores in the eye (eg, lipofuscin) that emit light in a range from ~540 to >700 nm are excited and autofluorescence can be detected. The FAF signal is mainly derived from lipofuscin and its dominant fluorophore N-retinylidene-N-retinyl-ethanolamine (A2E), accumulated in the retinal pigment epithelium (RPE).3 The Optomap imaging device differs from other FAF detection devices, as it uses a green laser for excitation and detects retinal autofluorescence within the yellow–orange–red range. One potential advantage of using the 532-nm wavelength for excitation is that the FAF signal may be more specific for lipofuscin, as interference from collagen autofluorescence may be reduced, compared with excitation of FAF at lower wavelengths (eg, 488 nm, as used in the Heidelberg HRA 2 FAF detection system [Heidelberg Engineering, Heidelberg, Germany]).1,3,4 The emitted FAF signal of the retina is detected from a raster scan and a bright-band detector for 570 to 780 nm. In addition to the detection of wide-field FAF, the Optomap P200Tx allows viewing of the two separate images from the red and green laser scans or both scans superimposed to yield semirealistic color imaging. For both fundus imaging and wide-field FAF, only a small optical pass of ≥2 mm is required and the specific mirror design of the Optomap P200AF prototype allows wide-field images of approximately 180°–200° to be obtained without pupil dilation. 2 The optical resolution of the specific instrument used in this study was 3900 × 3072 pixels, resulting in approximately 17–22 pixels per degree. The acquisition time was 0.25 seconds per scan.
Here we report our initial experience with Optomap wide-field FAF images in four patients with chorioretinitis.
Case 1
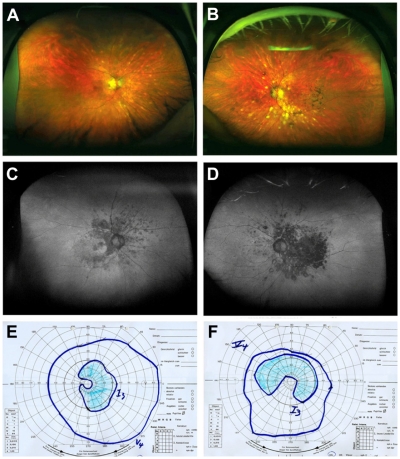
A 62-year-old man with human leukocyte antigen serotype A29 (HLA-A29)-positive birdshot chorioretinopathy and a history of blurred vision in both eyes came for a control visit to our uveitis clinic. Best-corrected visual acuity (BCVA) of the right and left eyes was 20/20 and 20/25. The intraocular pressure (IOP) was 15 mmHg in the right eye and 17 mmHg in the left eye. Slit-lamp examination revealed minimal anterior segment inflammation. Fundus examination of both eyes showed cream-colored and depigmented spots (Figure 1A and B). Visual fields were measured by Goldmann manual perimetry (scotoma right eye: temporal 35°, nasal 5°, superior 30°, inferior 35°, left eye: temporal 55°, nasal 35°, superior 35°, inferior 0°). The visual fields revealed paracentral scotoma in the upper temporal area in the right eye and an arcuate scotoma extending from the blind spot nasally in the left eye. A full clinical examination and Optomap FAF imaging were performed. Areas of reduced FAF corresponded to the visual field defects (Figure 1C–F). In the right eye, the defect was C-shaped around the macula and the peripapillary region, extending temporally. The left eye showed a partial loss of autofluorescence centrally and absolute confluent defects below the macula. Areas of reduced FAF corresponded markedly with visual field losses and choroidal damage.
Figure 1.
Case 1: (A, B, C, and D) Optomap color fundus imaging (A and B) and wide-field FAF (C and D) of the right (A and C) and left eye (B and D). (E and F) corresponding visual fields measured with Goldmann manual perimetry. The nasal paracentral scotoma of the right eye, and the large scotoma extending from the blind spot nasally on the left eye correspond well with the areas of reduced FAF.
Abbreviation: FAF, fundus autofluorescence.
Case 2
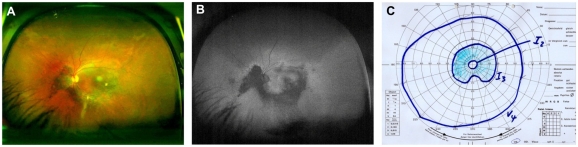
A 48-year-old man suffering from visual loss due to Vogt–Koyanagi–Harada syndrome in both eyes came for a follow-up visit to our uveitis clinic. He was under oral cyclosporine treatment (200 mg/day) and had pars plana vitrectomy with silicone oil due to exudative retinal detachment on his right eye one year prior to the visit. The left eye had been treated intravitreally with triamcinolone (4 mg) 19 months prior. On examination, the visual acuity was count fingers on the right eye and 20/100 on the left eye. Routine examination revealed an IOP of 15 mmHg in the right and 18 mmHg in the left. Slit-lamp examination of the anterior chamber and vitreous of both eyes was normal. Fundus examination of the right eye revealed a whitish central lesion under silicone oil. The left eye showed multiple, deep creamy-yellow choroidal lesions at the posterior pole and an old paracentral scar (Figure 2A). The visual field loss of the left eye showed a scotoma in the superior field sparing the macula. Corresponding to the visual field (scotoma left eye: temporal 20°, nasal 15°, superior 15°, inferior 0°), a loss of FAF was seen extending from the papilla below and temporal to the macula (Figure 2B and C).
Figure 2.
Case 2: (A and B) Optomap color fundus imaging (A) and wide-field FAF (B) of the right eye. (C) Corresponding visual field measured with Goldmann manual perimetry.
Abbreviation: FAF, fundus autofluorescence.
Case 3
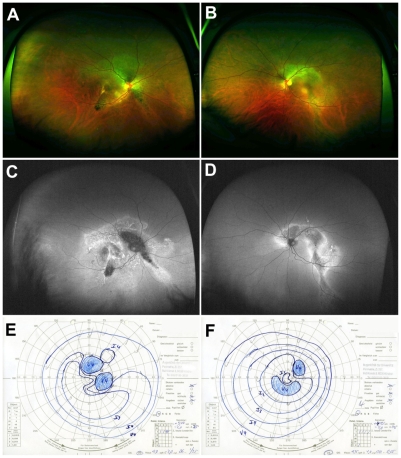
A 52-year-old patient was diagnosed with Vogt–Koyanagi–Harada syndrome five years ago and was under systemic infliximab treatment (5 mg/kg) for three months. At the time of presentation, the patient reported a change in visual acuity after several weeks. His BCVA was finger count in the right eye and 20/400 in the left eye. The IOP in both eyes was 13 mmHg. Slit-lamp examination showed a bilateral anterior granulomatous uveitis with keratic precipitates and few cells and flare in the anterior chamber’s aqueous fluid. Fundus examination of both eyes revealed moderate vitritis, choroidal folds, macular thickening, and perivascular edema. FAF revealed peripapillary arc-shaped areas of hypofluorescence extending from the central pole, surrounded by hyperfluorescent borders (Figure 3C and D). Findings from FAF, especially regarding the hypofluorescence areas, showed correspondence to relative scotoma in visual field obtained by Goldmann perimetry (scotoma right eye: temporal 20°, nasal 20°, superior 20°, inferior 0°, left eye: temporal 20°, nasal 25°, superior 20°, inferior 20°) (Figure 3).
Figure 3.
Case 3: (A, B, C, and D) Optomap color imaging (A and B), wide-field FAF (C and D) of the right and left eye, and corresponding visual fields (E and F). In both eyes peripapillary arc-shaped areas of hypofluorescence extending from the central pole, surrounded by hyperfluorescent borders can be seen in Optomap FAF (C and D).
Abbreviation: FAF, fundus autofluorescence.
Case 4
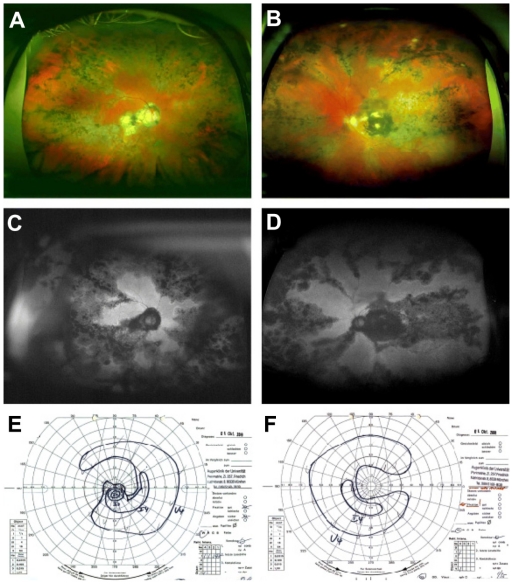
A 64-year-old man presented with complaints of decreased visual acuity in the right eye for four days secondary to a relapse of his long-standing history of choroiditis. At the time of presentation, no medication was taken. Visual acuity of the right and left eyes was 20/200 and 10/200. The IOP was 10 mmHg in the right eye and 12 mmHg in the left eye. Both eyes showed normal anterior chamber findings on slitlamp examination. Multiple old cells were seen in the vitreous. Fundus examination on both eyes showed pigmented scars in the area of the posterior pole and pigmented scars in the periphery with no signs of active inflammation (Figure 4A and B). Visual field testing on both eyes revealed central and nasal scotoma corresponding to the defects noted upon FAF examination (scotoma right eye: temporal 20°, nasal 30°, superior 30°, inferior 5°, left eye: temporal 15°, nasal 30°, superior 20°, inferior 5°). The autofluorescence images showed a distinctive geographic configuration that originated peripapillary and in the macular area and extended centrifugally (Figure 4C and D). The areas of RPE atrophy corresponded to the visual field as measured by Goldmann perimetry, presenting a central scotoma and decreased peripheral field of vision (Figure 4C–F).
Figure 4.
Case 4: (A, B, C, and D) Optomap color imaging (A and B), wide-field FAF (C and D) of the right eye and left, and corresponding visual fields (E and F). In both eyes, pigmented scars at the posterior pole and the periphery can be seen in Optomap color fundus imaging. FAF reveals distinct serpiginous and geographic areas of reduced FAF in both eyes. These findings correspond to visual field defects found in Goldmann perimetry.
Abbreviation: FAF, fundus autofluorescence.
Discussion
FAF has proven to be a valuable tool for the detection of RPE pathologies in several diseases. FAF has been used for both detection and monitoring of geographic atrophy secondary to age-related macular degeneration, diabetic maculopathy, and hereditary retinal disorders such as Stargardt disease.1,5–7 In addition, FAF has been shown to be an appropriate technique to detect inflammatory damage in chorioretinitis patients.7–9 Especially in patients with age-related macular degeneration, irregularities in central FAF have been correlated with a decrease in visual acuity.1 However, peripheral changes in the RPE often occur as well, potentially resulting in visual field defects. Detection of FAF using conventional imaging devices is usually limited to the posterior pole, as only 30° to 60° of the retina can be imaged in one scan.1 Therefore, only limited data regarding peripheral FAF and potential correlations to visual field defects are available.
The Optomap imaging system allows detection of FAF for up to 200° by using a green laser (532 nm) for excitation of FAF and a broad-band detector within the yellow–orange–red range.2 These features potentially result in more specific detection of lipofuscin-associated FAF, as interference from collagen autofluorescence may be less than when using lower excitation wavelengths.1,3,4 These unique properties of the Optomap P200Tx system allow better detection of FAF related to peripheral RPE alterations and may provide additional information regarding RPE-related retinal dysfunction of the peripheral retina.
Here we describe the use of this wide-field FAF imaging technique in four patients with chorioretinitis. Birdshot chorioretinopathy, and Vogt–Koyanagi–Harada syndrome are clinically well-characterized uveitis entities resulting in atrophic damage to the choriocapillaris, pigment epithelium, and adjacent outer layers of the retina, and specific changes in FAF have been described.8,9 This damage causes focal loss of visual acuity in the affected areas. Previous studies have investigated autofluorescence of retinal pigment at infrared wavelengths as well as wavelengths in the blue (excitation) and green (emission) spectra. Using the Optomap P200Tx, we investigated the autofluorescence with a green light excitation at 532 nm and emission at 570 to 780 nm. We found deep defects of autofluorescence in the damaged retinal areas. The four patients with focal loss of autofluorescence underwent visual field testing, which showed deep scotomas. Comparing the images from FAF detection with the visual fields, we found high concordance.
In the presented four cases, changes in FAF are closely related to certain inflammatory retinal changes. Hyperfluorescent areas in FAF have been described mainly as a result of hyperplastic pigment epithelium, whereas hypofluorescent areas seem to be closely correlated to decreased RPE activity and atrophy for example as a result of scarring from chronic or previous inflammation. Studies have demonstrated in many other diseases that loss of autofluorescence might indicate loss of function, which sometimes is irreversible.1,3,6,9,10 Findings from these four patients indicate that the Optomap P200Tx prototype might detect defective autofluorescence corresponding closely with loss of function. If this holds true in further studies, an additional tool is available that can detect damage and possibly new areas of damage after relapses. Wide-field FAF imaging potentially provides a noninvasive technique to monitor areas of old or new retinal inflammatory activity and may provide additional information to slit-lamp examination or visual field examination alone. An additional advantage of the Optomap P200Tx is that only a minimal optical pass (≥2 mm) is required. Therefore, wide-field FAF imaging can be obtained quick and easily even in eyes with undilated pupils. In addition, due to the SLO principle, the Optomap P200Tx provides good quality images even in eyes with media opacities due to intraocular inflammation.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Schmitz-Valckenberg S, Bültmann S, Dreyhaupt J, Bindewald A, Holz FG, Rohrschneider K. Fundus autofluorescence and fundus perimetry in the junctional zone of geographic atrophy in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45(12):4470–4476. doi: 10.1167/iovs.03-1311. [DOI] [PubMed] [Google Scholar]
- 2.Kernt M, Schaller UC, Stumpf C, Ulbig MW, Kampik A, Neubauer AS. Choroidal pigmented lesions imaged by ultra-wide-field scanning laser ophthalmoscopy with two laser wavelengths (Optomap) Clin Ophthalmol. 2010;4:829–836. doi: 10.2147/opth.s11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammer M, Königsdörffer E, Liebermann C, et al. Ocular fundus autofluorescence observations at different wavelengths in patients with age-related macular degeneration and diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2008;246(1):105–114. doi: 10.1007/s00417-007-0639-9. [DOI] [PubMed] [Google Scholar]
- 4.Schweitzer D, Schenke S, Hammer M, et al. Towards metabolic mapping of the human retina. Microsc Res Tech. 2007;70(5):410–419. doi: 10.1002/jemt.20427. [DOI] [PubMed] [Google Scholar]
- 5.Boon CJ, Jeroen Klevering B, Keunen JE, Hoyng CB, Theelen T. Fundus autofluorescence imaging of retinal dystrophies. Vision Res. 2008;48(26):2569–2577. doi: 10.1016/j.visres.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Fleckenstein M, Charbel Issa P, Fuchs HA, et al. Discrete arcs of increased fundus autofluorescence in retinal dystrophies and functional correlate on microperimetry. Eye (Lond) 2009;23(3):567–575. doi: 10.1038/eye.2008.59. [DOI] [PubMed] [Google Scholar]
- 7.Giuliari G, Hinkle DM, Foster CS. The spectrum of fundus autofluorescence findings in birdshot chorioretinopathy. J Ophthalmol. 2009;2009:567693. doi: 10.1155/2009/567693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasconcelos-Santos DV, Sohn EH, Sadda S, Rao NA. Retinal pigment epithelial changes in chronic Vogt-Koyanagi-Harada disease: fundus autofluorescence and spectral domain-optical coherence tomography findings. Retina. 2010;30(1):33–41. doi: 10.1097/IAE.0b013e3181c5970d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeh S, Forooghian F, Wong WT, et al. Fundus autofluorescence imaging of the white dot syndromes. Arch Ophthalmol. 2010;128(1):46–56. doi: 10.1001/archophthalmol.2009.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vujosevic S, Casciano M, Pilotto E, Boccassini B, Varano M, Midena E. Diabetic macular edema: fundus autofluorescence and functional correlations. Invest Ophthalmol Vis Sci. 2011;52(1):442–448. doi: 10.1167/iovs.10-5588. [DOI] [PubMed] [Google Scholar]