Table 1.
Enantioselective Allylation of Cyclic Enones.
| Enonea | Yieldb | Product | ee |
|---|---|---|---|
1
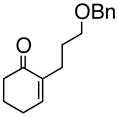 1a |
84% |
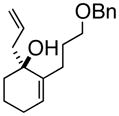 3a |
98% ee |
2
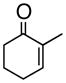 1bc,d |
46% |
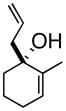 3b |
97% ee |
3
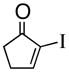 1c |
92% |
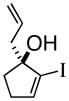 3c |
93% ee |
4
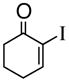 1d |
95% |
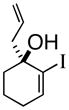 3d |
92% ee |
5
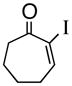 1e |
89% |
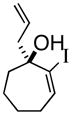 3e |
85% ee |
Additions were carried out using 5 mol % (S)-3,3′-dibromobinol.
Yields are for pure isolated substances.
Addition was effected using 5 mol % (R)-3,3′-dibromobinol.
Both the starting material and the product were volatile.
