Table 2.
KH-Mediated oxy-Cope Rearrangement.
| Alcohola | Productb | Yieldc |
|---|---|---|
1
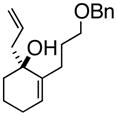 3a |
 4a |
70% |
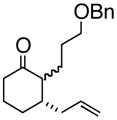
2
 3bd |
 4b |
64% |
3
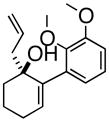 3dAre |
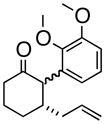 4c |
89% |
4
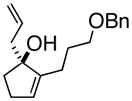 3cAlke |
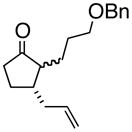 4d |
80% |
5
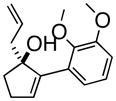 3cAre |
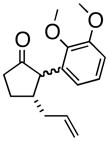 4e |
67% |
6
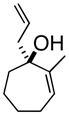 3eMef |
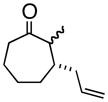 4f |
52% |
Oxy-Cope rearrangement was carried out with dicyclohexyl-18-crown-6 and KH in THF.
Products were a mixture of α-epimers.
Yields are for pure isolated substances.
t-BuOK was used for the rearrangement.
Prepared by Stille coupling of the corresponding iodoalkene.
Prepared by Kumada coupling of the corresponding alkene.
