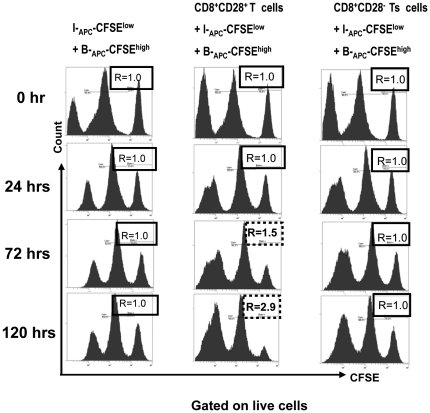
Figure 5. Cytotoxicity does not contribute to the suppression of proliferation by the in vitro expanded CD8+CD28− T cells.
A CFSE based cytotoxicity assay was set up as follows: target cells were the original priming APCs (for generating the CD8+CD28− T cells) labeled with a high concentration (2.0 µM) of CFSE (B-APC-CFSEhigh), or APCs from an HLA-A, -B, and -DR mismatched indifferent individual labeled with a low concentration (0.2 µM) of CFSE (I-APC-CFSElow). A 1∶1 mixture of I-APC-CFSElow and B-APC-CFSEhigh cells were plated either by themselves (left panels), or with the same number of control (CD8+CD28+, middle panels) or putative (CD8+CD28−, right panels) effector cells in 96 U-bottom plates in triplicates. At 24, 72, and 120 hr of culture, cells were collected and analyzed by FACS to enumerate CFSElow and CFSEhigh cells. The ratio of cell numbers of I-APC-CFSElow over B-APC-CFSEhigh was calculated as “R” for all time points. An increase in the value of R indicates specific killing of B-APC-CFSEhigh cells by the effector cells, whereas a stable value of R at 1.0 indicates no specific killing of B-APC-CFSEhigh cells by the effector cells. Data shown are representative of two independent experiments.