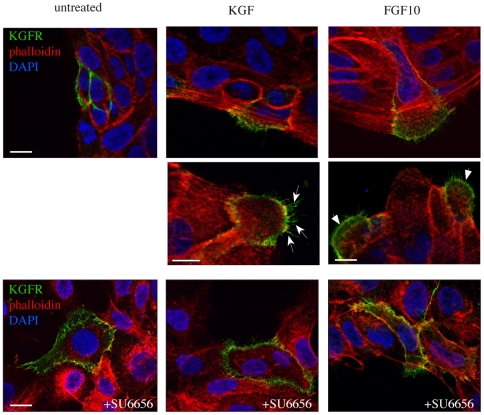
Figure 2. Src activity is responsible for KGFR polarization at the leading edge of migrating cells.
HaCaT cells transiently transfected with the human KGFR (HaCaT KGFR) were treated with KGF or FGF10 and then immunostained at 4°C with an anti-Bek polyclonal antibodies, which recognize the extracellular portion of the receptor, and do not compete with the ligands for binding. Cells were then fixed, permeabilized, and stained with TRITC-conjugated phalloidin to visualize the actin cytoskeleton organization. Nuclei were stained with DAPI. Images are obtained, as described in Materials and Methods, by serial optical sectioning and 3D reconstruction of a selection of three out of the total number of sections: the selected sections are all central and crossing the nucleus visualized by DAPI. Results show that, in peripheral untreated HaCaT KGFR cells, the KGFR signal appears homogeneously distributed on the entire plasma membrane and the actin is not organized in typical migratory structures. After either KGF or FGF10 treatment, the KGFR signal appears polarized at the leading edge of migrating cells, where actin cytoskeleton is mainly organized in filopodia (arrows), but also in ruffles and small lamellipodia (arrowheads). In the presence of the Src inhibitor SU6656, the KGFR signal remains uniformly distributed on the plasma membrane, also upon ligand stimulation, and the actin is organized in few thin filopodia distributed over the entire cell surface. Bars: 10 µm.