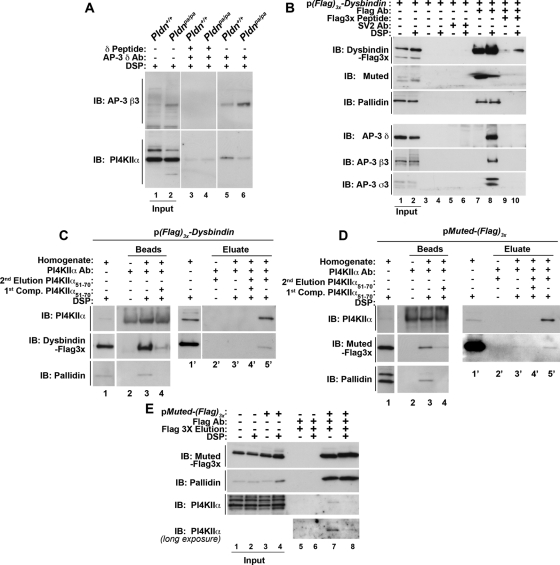
FIGURE 1:
Dysbindin coprecipitates BLOC-1 subunits, AP-3 complexes, and PI4KIIα. (A) Wild-type (Pldn+/+, odd lanes) or pallidin-deficient (Pldnpa/pa, even lanes) mouse skin primary culture fibroblasts were treated with DSP, detergent solubilized, and extracts precipitated with magnetic beads with antibodies against AP-3 delta (lanes 3–6) in either the absence (lanes 5 and 6) or presence (lanes 3 and 4) of delta antigenic peptide as an immunoprecipitation control. (B) SH-SY5Y stably expressing triple FLAG dysbindin treated in the absence or presence of DSP were solubilized in detergent and extracts precipitated with magnetic beads alone as controls (lanes 3–6), with antibodies against FLAG tag (lanes 7–10). Precipitations were performed in the absence or presence of an excess of FLAG peptide (lanes 9 and 10). An irrelevant antibody (SV2, lanes 5 and 6) was used to confirm specificity. (C) SH-SY5Y FLAG dysbindin or (D) SH-SY5Y FLAG muted cell extracts were precipitated with PI4KIIα antibodies (lanes 3, 4, and 3′–5′) in either the absence or presence of PI4KIIα peptide 51-70 to outcompete binding of PI4KIIα complexes to beads (first Competition [Comp.], lanes 4 and 4′). Protein complexes bound to beads were eluted with SDS–PAGE sample buffer (lanes 2-–4). The band detected in PI4KIIα blots in lanes 1 and 4 corresponds to the rabbit anti-PI4KIIα immunoglobulin G also used for immunoprecipitation. Alternatively, PI4KIIα protein complexes were eluted from beads with buffer in either the absence (lane 3′) or presence of 200 μM PI4KIIα peptide 51-70 (second elusion, lane 5′). (E) Untransfected SH-SY5Y (lanes 1, 2, 5, and 6) or SH-SY5Y FLAG muted cells treated in the absence or presence of DSP were solubilized in detergent and extracts were precipitated with FLAG antibodies (lanes 5–8), and FLAG muted protein complexes were eluted from beads with 200 μM FLAG peptide (lanes 5–8). Note that PI4KIIα coprecipitates with FLAG-tagged muted even in the absence of DSP (lane 7). Specificity was determined by using cell extracts from nontransfected cells (lanes 5 and 6). Immune complexes resolved by SDS–PAGE were analyzed by immunoblot with antibodies against FLAG, the BLOC-1 subunits pallidin and muted, AP-3 subunits (δ, β3, σ3), and PI4KIIα. Inputs are 10%, and in B–D inputs are lanes 1 and 1′.