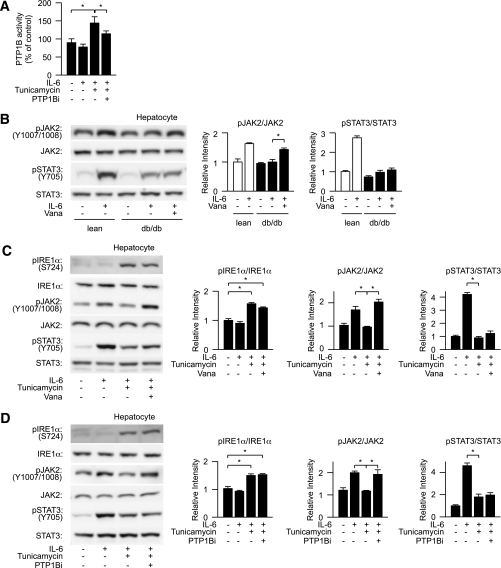
FIG. 4.
Inhibition of protein tyrosine phosphatase improves ER stress–induced suppression of JAK2 phosphorylation. A: Lean mouse–derived hepatocytes were pretreated with tunicamycin and PTP1B inhibitor followed by stimulation with IL-6 and analyzed for the effect of ER stress on cellular PTP1B activity. PTP1B activity was expressed as a percentage of that of the controls. B: db/db mouse–derived hepatocytes were treated with sodium orthovanadate (Vana) and analyzed for the levels of IL-6–dependent JAK2 phosphorylation and STAT3 phosphorylation (left). Quantitation of phosphorylation of IRE1α and STAT3 is normalized to total IRE1α and STAT3, respectively, and is represented as mean ± SE (right). *P < 0.05; open bar, lean hepatocyte; closed bar, db/db hepatocytes. C and D: Lean mouse–derived hepatocytes were pretreated with tunicamycin and sodium orthovanadate (Vana) or a PTP1B inhibitor (PTP1Bi) followed by stimulation with IL-6 and analyzed for the levels of phosphorylation of IRE1α and tyrosine phosphorylation of JAK2 and STAT3 (left). Quantitation of IRE1α, JAK2, and STAT3 phosphorylation levels is normalized to total IRE1α, JAK2, and STAT3, respectively, and is represented as mean ± SE (right). *P < 0.05.