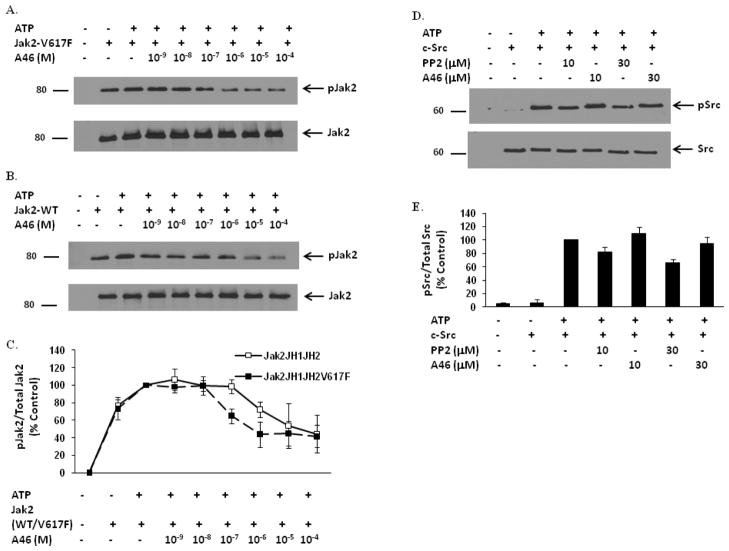
Figure 1. A46 selectively inhibits Jak2-V617F protein in a cell-free system.
Recombinant Jak2-JH1+JH2-V617F (mutant) (A) and Jak2-JH1+JH2 (wild-type) (B) proteins were incubated in a kinase reaction buffer, either with DMSO or with the indicated doses of A46. The kinase reactions were then separated on an SDS-PAGE and immunoblotted with a phospho-Jak2 (pY1007/pY1008) antibody (A & B, top panels). The membranes were stripped and reprobed with an anti-Jak2 antibody to demonstrate equal loading of protein across all lanes (A & B, bottom panels). Shown is one of four representative results for each. C. Densitometric analysis was done on four representative Western blots to quantify A46-mediated inhibition of Jak2-V617F and Jak2 wild-type phosphorylation at the Tyr1007 residue. The ratio of phosphorylated Jak2 to total Jak2 was expressed as % control and plotted as a function of treatment condition. Shown are the means ± S.D. of four independent experiments. D. Recombinant c-Src protein was incubated in a kinase reaction buffer, either with DMSO or with the indicated doses of PP2 and A46. The kinase reactions were then separated on an SDS-PAGE and immunoblotted with a phospho-Src (pY418) antibody (D, top panel) or with an anti-Src antibody to demonstrate equal loading of protein across all lanes (D, bottom panel). Shown is one of three representative results. E. Densitometric analysis was done on the Western blots to quantify A46-mediated inhibition of c-Src phosphorylation at the Tyr418 residue. The ratio of phosphorylated Src to total Src was expressed as % control and plotted as a function of treatment condition. Shown are the means ± S.D. of three independent experiments.