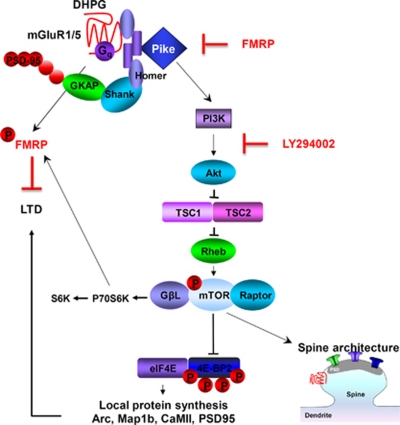
Figure 1.
Scheme showing the link between deficits in FMRP and overactivated mTOR. Our findings support a model where, in WT mice, FMRP represses PIKE, an upstream activator of PI3K signaling and identified target of FMRP, and thereby inhibits mTOR signaling. Upon activation, group I mGluRs act via Gq and Homer to bind PIKE and engage PI3K signaling, which activates mTOR. mTOR drives cap-dependent translation and local synthesis of synaptic proteins such as Arc, Map1b, CaMII, and PSD-95, critical to mGluR-LTD. In addition, mTOR regulates LIMK and cofilin, which promote spine morphogenesis. In mice lacking FMRP, PIKE is derepressed, resulting in overactivation of mTOR, accumulation of synaptic proteins, and exaggerated, protein synthesis-independent LTD. The PI3K inhibitor LY294002 corrects p-mTOR and restores DHPG sensitivity. A prediction of the model is that dysregulation of mTOR signaling contributes to the cognitive and social interaction deficits observed in humans with Fragile X.