Figure 3.
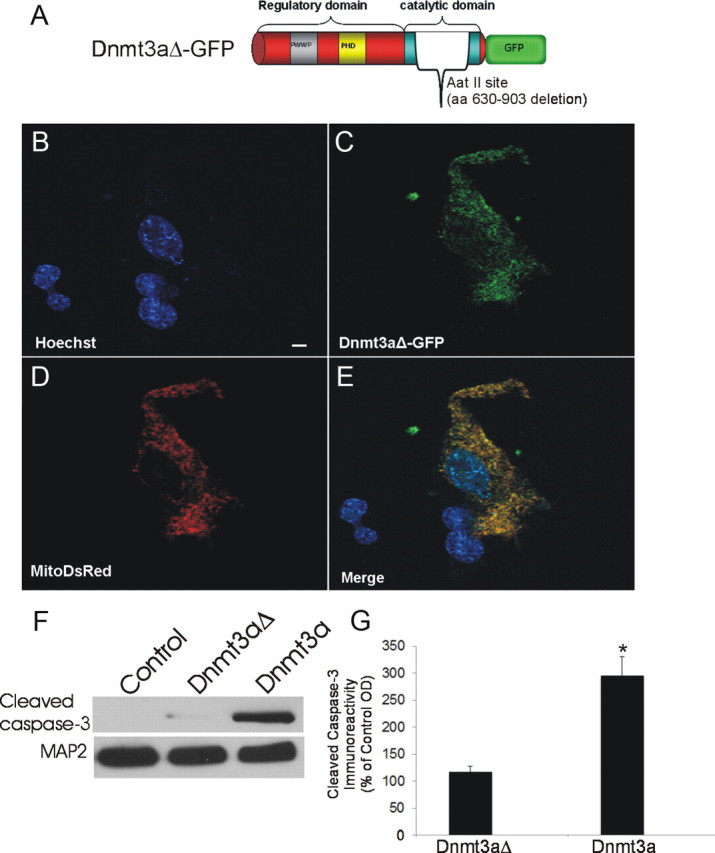
Mutant Dnmt3a-GFP localizes to mitochondria. A, Schematic diagram showing design of mutated Dnmt3a gene construct with a deleted catalytic domain. B–E, Cultured NSC34 cells were cotransfected with Dnmt3aΔ-GFP (C) and mitochondrial-targeted DsRed (D) plasmids and cultured for 48 h to 16 d. Fixed NSC-34 cells were stained with Hoechst 33258 (B). Dnmt3aΔ-GFP was localized to the nucleus and cytoplasm (C). E, Cytoplasmic Dnmt3aΔ-GFP showed near-complete localization to mitochondria (yellow). Scale bar (in B), 7 μm. F, NSC34 cells transfected with catalytic domain mutant Dnmt3a did not show accumulation of cleaved caspase-3 by Western blotting as did cells transfected with wild-type Dnmt3a. MAP2 immunoreactivity was used as a protein loading control. G, Western blot quantification of cleaved caspase-3 immunoreactivity in lysates of NSC34 cells transfected with catalytic domain mutant Dnmt3a (Dnmt3aΔ) and wild-type Dnmt3a compared with mock-transfected control cells. Values (percentage of control) are mean ± SD of three experiments. Asterisk indicates significant difference (p < 0.01) compared with control and Dnmt3aΔ cells.
