Abstract
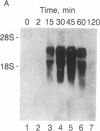
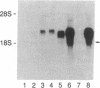
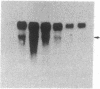
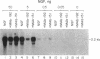
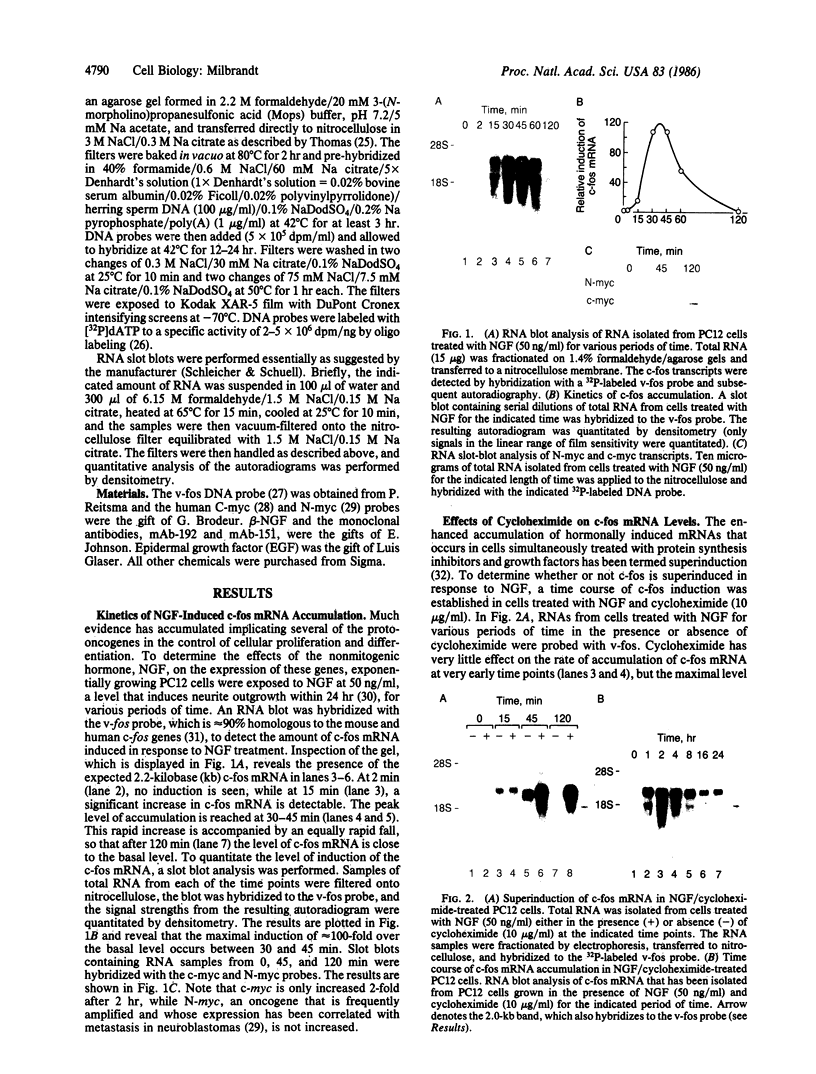
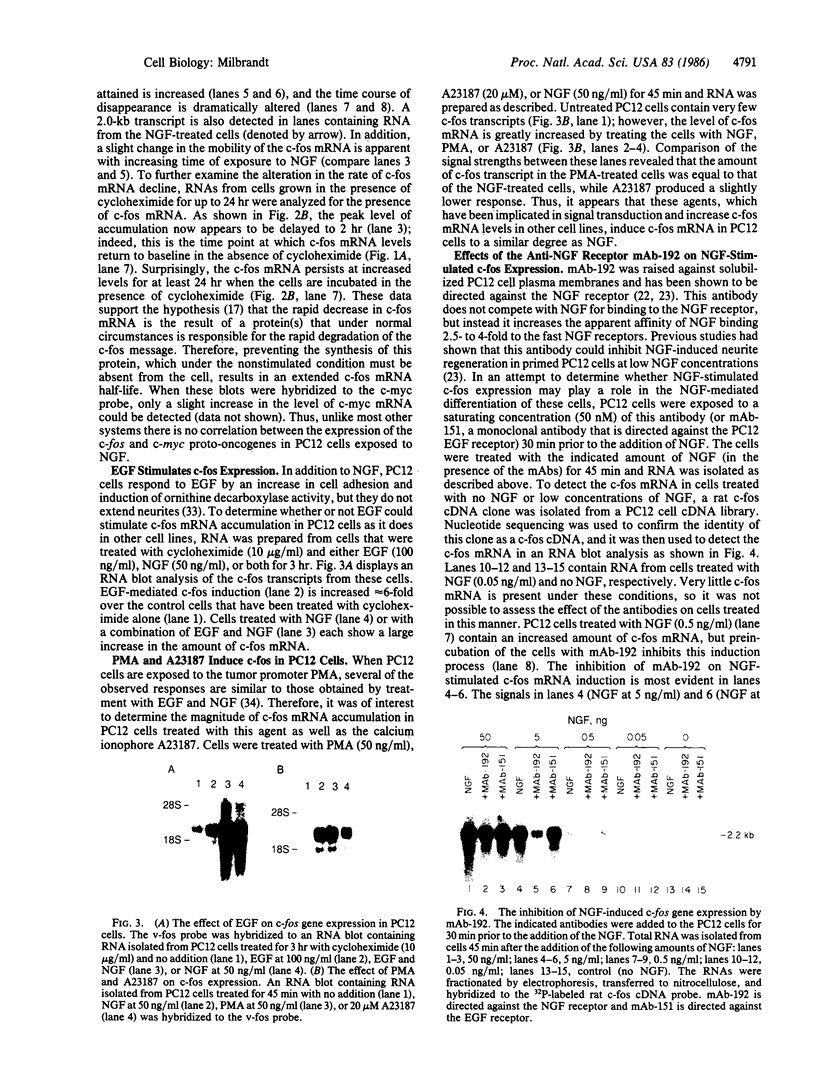
The nerve growth factor (NGF)-mediated increase in c-fos gene expression in the rat pheochromocytoma PC12 cell line has been investigated. NGF treatment of PC12 cells results in an increased level of c-fos mRNA within 15 min. An approximately 100-fold increase in the level of c-fos mRNA occurs 30-45 min after exposure to NGF and the c-fos mRNA concentration returns to its basal level 2 hr after NGF treatment. Thus, the half-life of this RNA transcript is extremely short. In the presence of cycloheximide, the c-fos gene is superinduced and the increased level of c-fos mRNA persists for at least 24 hr. The induction of c-fos gene expression was further studied by utilizing a monoclonal antibody (mAb-192) that is directed against the NGF receptor but does not compete with NGF for binding to the receptor. Treatment of the cells with mAb-192 inhibits the NGF-stimulated elevation of c-fos mRNA, suggesting that the antibody may interfere with the receptor's ability to generate the signal required to stimulate the transcription of this gene. NGF is not the only agent capable of inducing c-fos gene expression in these cells; epidermal growth factor, the tumor promoter phorbol 12-myristate 13-acetate, and the calcium ionophore A23187, agents that induce the c-fos gene in other cell lines, are also effective in PC12 cells. The mRNA for the nuclear protein fos is rapidly induced by NGF and other agents to which PC12 cells respond. This supports the hypothesis that the fos gene product may play a role in signal transduction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres R. Y., Jeng I., Bradshaw R. A. Nerve growth factor receptors: identification of distinct classes in plasma membranes and nuclei of embryonic dorsal root neurons. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2785–2789. doi: 10.1073/pnas.74.7.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Burckhardt J., Curran T., Müller R. Stimulation and inhibition of growth by EGF in different A431 cell clones is accompanied by the rapid induction of c-fos and c-myc proto-oncogenes. EMBO J. 1985 May;4(5):1193–1197. doi: 10.1002/j.1460-2075.1985.tb03759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C., Schwab M., Varmus H. E., Bishop J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984 Jun 8;224(4653):1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- Burstein D. E., Greene L. A. Evidence for RNA synthesis-dependent and -independent pathways in stimulation of neurite outgrowth by nerve growth factor. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6059–6063. doi: 10.1073/pnas.75.12.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler C. E., Parsons L. M., Hosang M., Shooter E. M. A monoclonal antibody modulates the interaction of nerve growth factor with PC12 cells. J Biol Chem. 1984 Jun 10;259(11):6882–6889. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chun L. L., Patterson P. H. Role of nerve growth factor in the development of rat sympathetic neurons in vitro. II. Developmental studies. J Cell Biol. 1977 Dec;75(3):705–711. doi: 10.1083/jcb.75.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Zullo J., Verma I. M., Stiles C. D. Expression of the c-fos gene and of an fos-related gene is stimulated by platelet-derived growth factor. Science. 1984 Nov 30;226(4678):1080–1082. doi: 10.1126/science.6093261. [DOI] [PubMed] [Google Scholar]
- Curran T., Morgan J. I. Superinduction of c-fos by nerve growth factor in the presence of peripherally active benzodiazepines. Science. 1985 Sep 20;229(4719):1265–1268. doi: 10.1126/science.4035354. [DOI] [PubMed] [Google Scholar]
- Curran T., Peters G., Van Beveren C., Teich N. M., Verma I. M. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982 Nov;44(2):674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano P. S., Schweitzer J. B., Taniuchi M., Johnson E. M., Jr Selective destruction of nerve growth factor receptor-bearing cells in vitro using a hybrid toxin composed of ricin A chain and a monoclonal antibody against the nerve growth factor receptor. J Cell Biol. 1985 Sep;101(3):1107–1114. doi: 10.1083/jcb.101.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- End D., Tolson N., Yu M. Y., Guroff G. Effects of 12-0-Tetradecanoylphorbol-13-acetate (TPA) on rat pheochromocytoma (PC12) cells: interactions with epidermal growth factor and nerve growth factor. J Cell Physiol. 1982 May;111(2):140–148. doi: 10.1002/jcp.1041110204. [DOI] [PubMed] [Google Scholar]
- Garrels J. I., Schubert D. Modulation of protein synthesis by nerve growth factor. J Biol Chem. 1979 Aug 25;254(16):7978–7985. [PubMed] [Google Scholar]
- Greenberg M. E., Greene L. A., Ziff E. B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985 Nov 15;260(26):14101–14110. [PubMed] [Google Scholar]
- Greene L. A., Liem R. K., Shelanski M. L. Regulation of a high molecular weight microtubule-associated protein in PC12 cells by nerve growth factor. J Cell Biol. 1983 Jan;96(1):76–83. doi: 10.1083/jcb.96.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., McGuire J. C. Induction of ornithine decarboxylase by nerve growth factor dissociated from effects on survival and neurite outgrowth. Nature. 1978 Nov 9;276(5684):191–194. doi: 10.1038/276191a0. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry I. A., Stöckel K., Thoenen H., Iversen L. L. The retrograde axonal transport of nerve growth factor. Brain Res. 1974 Mar 15;68(1):103–121. doi: 10.1016/0006-8993(74)90536-8. [DOI] [PubMed] [Google Scholar]
- Huff K., End D., Guroff G. Nerve growth factor-induced alteration in the response of PC12 pheochromocytoma cells to epidermal growth factor. J Cell Biol. 1981 Jan;88(1):189–198. doi: 10.1083/jcb.88.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. M., Jr, Gorin P. D., Brandeis L. D., Pearson J. Dorsal root ganglion neurons are destroyed by exposure in utero to maternal antibody to nerve growth factor. Science. 1980 Nov 21;210(4472):916–918. doi: 10.1126/science.7192014. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Schubert D., Verma I. M. Induction of the proto-oncogene fos by nerve growth factor. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7330–7334. doi: 10.1073/pnas.82.21.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R., Booker B. DESTRUCTION OF THE SYMPATHETIC GANGLIA IN MAMMALS BY AN ANTISERUM TO A NERVE-GROWTH PROTEIN. Proc Natl Acad Sci U S A. 1960 Mar;46(3):384–391. doi: 10.1073/pnas.46.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire J. C., Greene L. A., Furano A. V. NGF stimulates incorporation of fucose or glucosamine into an external glycoprotein in cultured rat PC12 pheochromocytoma cells. Cell. 1978 Oct;15(2):357–365. doi: 10.1016/0092-8674(78)90004-1. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Müller D., Guilbert L. Differential expression of c-fos in hematopoietic cells: correlation with differentiation of monomyelocytic cells in vitro. EMBO J. 1984 Aug;3(8):1887–1890. doi: 10.1002/j.1460-2075.1984.tb02063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Tremblay J. M., Cline M. J., Verma I. M. Differential expression of cellular oncogenes during pre- and postnatal development of the mouse. Nature. 1982 Oct 14;299(5884):640–644. doi: 10.1038/299640a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Otten U., Schwab M., Gagnon C., Thoenen H. Selective induction of tyrosine hydroxylase and dopamine beta-hydroxylase by nerve growth factor: comparison between adrenal medulla and sympathetic ganglia of adult and newborn rats. Brain Res. 1977 Sep 16;133(2):291–303. doi: 10.1016/0006-8993(77)90765-x. [DOI] [PubMed] [Google Scholar]
- Reitsma P. H., Rothberg P. G., Astrin S. M., Trial J., Bar-Shavit Z., Hall A., Teitelbaum S. L., Kahn A. J. Regulation of myc gene expression in HL-60 leukaemia cells by a vitamin D metabolite. Nature. 1983 Dec 1;306(5942):492–494. doi: 10.1038/306492a0. [DOI] [PubMed] [Google Scholar]
- Richter-Landsberg C., Greene L. A., Shelanski M. L. Cell surface Thy-1-cross-reactive glycoprotein in cultured PC12 cells: modulation by nerve growth factor and association with the cytoskeleton. J Neurosci. 1985 Feb;5(2):468–476. doi: 10.1523/JNEUROSCI.05-02-00468.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley P. J., Keith C. H., Shelanski M. L., Greene L. A. Pressure microinjection of nerve growth factor and anti-nerve growth factor into the nucleus and cytoplasm: lack of effects on neurite outgrowth from pheochromocytoma cells. J Neurosci. 1983 Jul;3(7):1488–1494. doi: 10.1523/JNEUROSCI.03-07-01488.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton D. L., Reichardt L. F. Expression of the beta-nerve growth factor gene correlates with the density of sympathetic innervation in effector organs. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7951–7955. doi: 10.1073/pnas.81.24.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi M., Johnson E. M., Jr Characterization of the binding properties and retrograde axonal transport of a monoclonal antibody directed against the rat nerve growth factor receptor. J Cell Biol. 1985 Sep;101(3):1100–1106. doi: 10.1083/jcb.101.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Curran T., Müller R., Verma I. M. Analysis of FBJ-MuSV provirus and c-fos (mouse) gene reveals that viral and cellular fos gene products have different carboxy termini. Cell. 1983 Apr;32(4):1241–1255. doi: 10.1016/0092-8674(83)90306-9. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Shooter E. M. The biology and mechanism of action of nerve growth factor. Annu Rev Biochem. 1982;51:845–868. doi: 10.1146/annurev.bi.51.070182.004213. [DOI] [PubMed] [Google Scholar]
- Yip H. K., Grafstein B. Effect of nerve growth factor on regeneration of goldfish optic axons. Brain Res. 1982 Apr 29;238(2):329–339. doi: 10.1016/0006-8993(82)90108-1. [DOI] [PubMed] [Google Scholar]