Figure 4. PIP2 binding sites of TRPM6.
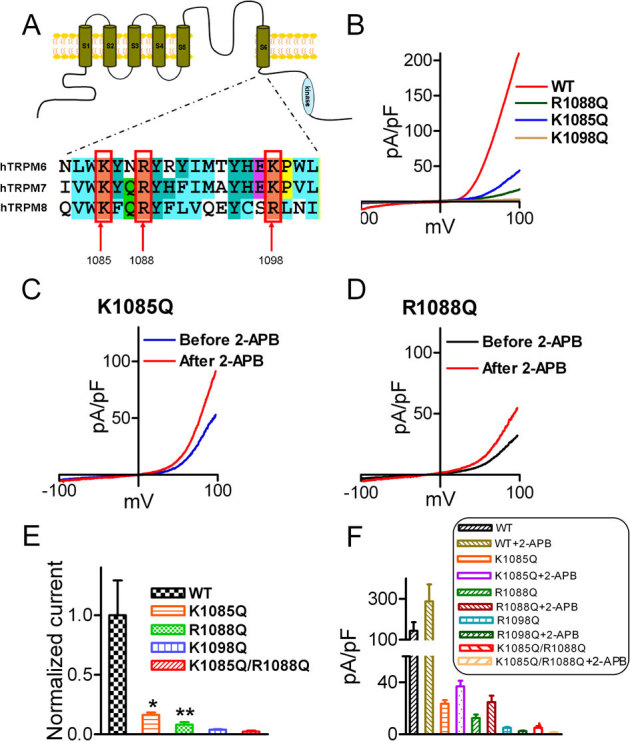
(A) Alignment of the TRP domain of TRPM6, TRPM7 and TRPM8. The highlighted residues in TRPM8 are the PIP2 binding sites. (B) Representative traces of WT-TRPM6 and mutants K1085Q, R1088Q, and K1098Q. (C–D) Effects of 2-APB on the mutants K1085Q and R1088Q. (E) Normalized mean current density of TRPM6 mutants in comparison with WT TRPM6 (n = 10 *p<0.05; ** P<0.01). Mutants K1098Q and the double mutants K1085Q/R1088Q did not produce any current. (F) Average changes of current amplitude by 2-APB (200 μM). 2-APB did not have any effect on the non-functional mutants K1098Q and K1085Q/R1088Q.
