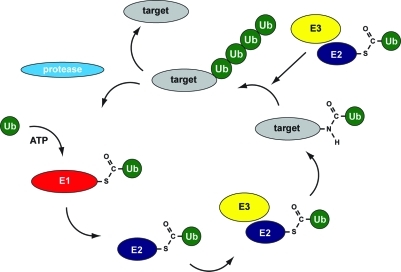
Figure 1.
Ubiquitylation cascade. Ubiquitin precursors are processed by proteases to generate mature ubiquitin containing a C-terminal diglycine motif for conjugation to target proteins. Three different classes of enzymes are involved: E1, E2, and E3 enzymes. Ubiquitin is coupled to the active site cysteine of the E1 enzyme in an adenosine 5′-triphosphate-dependent manner to form a thioester. Subsequently, transfer to the active site cysteine in an E2 enzyme occurs, and a novel thioester is formed. With the help of E3 enzymes, ubiquitin is coupled to lysines in target proteins via isopeptide bonds, and in a subsequent optional step, ubiquitin chains can be formed. Specific proteases remove ubiquitin from target proteins, and free ubiquitin becomes available for novel rounds of conjugation.