Figure 8.
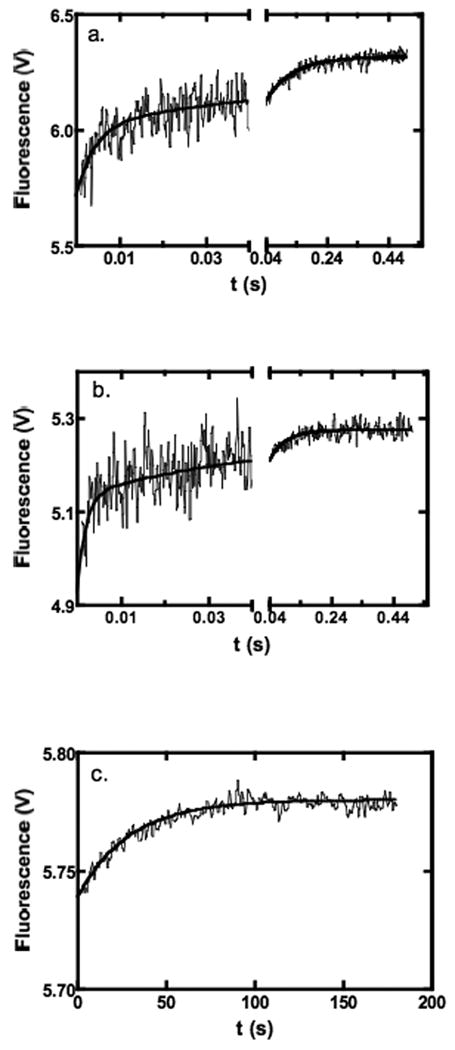
Examples of fluorescence stopped-flow traces showing the kinetics of MgTNP-ATP binding to pyruvate carboxylase at 30°C with 0.5 μM pyruvate carboxylase in 0.1M Tris-Cl, pH7.8 containing 20 mM NaHCO3. (a) Fast and intermediate phases of the reaction with 10 μM MgTNP-ATP (500 data points collected in the range 0-0.04 s and 500 data points collected in the range 0.04-1 s, average of 10 traces). Lines represent a fit to a double exponential equation, where data points up to 1 ms have been excluded from the fit (kobs1 = 234 ± 26 s-1, kobs2 = 11 ± 1 s-1). (b) Fast and intermediate phases of the reaction with 35 μM MgTNP-ATP (500 data points collected in the range 0-0.04 s and 500 data points collected in the range 0.04-0.5 s, average of 7 traces). Lines represent a fit to a double exponential equation, where data points up to 1 ms have been excluded from the fit (kobs1 = 475 ± 90 s-1, kobs2 = 19 ± 2 s-1). (c) Slow phase of the reaction with 20 μM MgTNP-ATP showing the 500 data points collected in the range 1-180s (average of 2 traces). The solid line represents a fit to a single exponential equation, where data points up to 3 s have been excluded from the fit (k = 0.035 ± 0.001 s-1).
